By Samantha Miller, President and Chief Scientist of Pure Analytics Cannabis Laboratory.
With the rise of the medical cannabis dispensary, several other types of businesses and service providers have evolved to help support their needs, including laboratories that test products for the medical cannabis industry. Prior to the existence of medical cannabis legalization, cannabis testing was restricted to analysis conducted as part of criminal investigation or within one of only a few research programs, such as the one at the University of Mississippi, overseen by Dr. Mahmoud ElSolhy, or, in Israel, that of Dr. Raphael Mechoulam, which perform government-sanctioned research.
Today the development and advancements of independent cannabis testing laboratories in states with medical and recreational cannabis legalization represent exciting progress both in the pursuit of medical-grade cannabis grown and processed to specified standards. Exciting, too, is the foundation of a powerful tool for the independent cannabis farmer to drive strategic breeding programs focused on isolating traits of interest and increasing potency in a predictive way using testing in the vegetative stage to assist selection of plants for breeding or growing. See “Cannabinoid Potency Testing as a Tool for the Farmer and Breeder”.
Dr. Mechoulam lecturing at the Hebrew University of Jerusalem
Several types of testing are performed by laboratories specializing in cannabis analytics to support the medical dispensary model, including cannabinoid potency testing; microbiological screening for molds, fungi, and bacteria; and pesticides testing that looks for residues of harmful chemicals that may have been applied during the growth cycle. As testing services and legalized cannabis have proliferated and the marketplace of products has quickly evolved and changed, so have the offerings by cannabis laboratories. Many laboratories now offer testing for flavors and fragrance compounds (known as terpenes) contained in cannabis. The gravitation toward chemically extracted concentrates known as wax, shatter, dabs, and more has also resulted in the demand for specific services such as residual solvent testing and testing for toxic impurities found in low-grade butane, propane, and CO2 gas often used to make wax. In regions where regulation is in keen focus, you will also find state-imposed requirements, such as in Nevada, mandating heavy-metals testing and residual fertilizer and nutrient testing.

The medical cannabis dispensary, Florin Wellness Center (FWC), is doing a brisk business in this photo snapped by Paul Clemons.
The first US cannabis lab opened in Colorado. Many others quickly followed, the majority in California where the burgeoning dispensary market saw the proliferation of hundreds of dispensaries within a single city, almost overnight. The most common type of testing performed by cannabis laboratories is cannabinoid potency testing. Potency testing provides medical cannabis patients with important information on the active compounds in their cannabis. This can help them select the right cannabis for them based on their specific ailment, telling them which active compounds are present and their relative amounts. Cannabinoids commonly tested for include THC, THCA, CBD, CBDA, CBN, CBC, and CBG, and soon will include THCV and CBDV, thanks to the efforts of chemical manufacturing companies supporting the specific needs of the cannabis testing industry.
American cannabis laboratories are limited in the cannabinoids they can legitimately include in their analysis, depending on which cannabinoids are available for purchase as certified reference standards from chemical standards companies in the USA. At the time of this book’s publication, the cannabinoid reference standards available in the USA for use by analysts include: THC, THCA, CBD, CBDA, CBG, CBN, CBDV, and CBC. These reference standards are purified cannabinoids in solution, and are used to calibrate the equipment used to measure potency. These certified reference solutions are what allow the chemist to identify and quantify the cannabinoids present in an extract. When equipment is properly calibrated using certified reference standards, two different labs should be able to measure the same extracted cannabis and provide a similar result. Some cannabis labs choose to report on cannabinoids based on theoretical data rather than verification of identity and amount based on the use of a certified chemical reference standard. Such a practice may be acceptable in a research setting but it is not acceptable in a patient-oriented setting where service providers should be operating under the same standards “as if” they were being regulated by the federal government and their analyses “certified.” However, in a crowded and highly competitive marketplace of service providers, many will “reach” to claim they can provide services or analyze for certain compounds such as exotic and rare cannabinoids that others cannot in an attempt to develop a competitive advantage in the cannabis-testing marketplace. If no one else is testing for a particular compound, there is likely a reason why, and the legitimacy of the analysis may be called into question. This leads us to the importance of selecting a service provider who can provide reliable and accurate results.

Various cannabis concentrates (left to right): wax, shatter, bubble-hash, and pressed kief (hash).
Selecting a Cannabis Laboratory
As dispensaries, patients, and farmers set out to choose the service provider who is right for them, it can quickly feel like wading through a confusing litany of opinions, marketing speak, and options. Having the facts at hand about testing processes and equipment relative to your own needs can help guide you to the best service provider for your needs.
Cannabinoid potency testing has been by far the most controversial during the infancy of the independent cannabis testing lab industry in the USA. It has led to many blind tests of service providers within a region by various groups either seeking to legitimize the testing process or rather to expose flaws in the practices of cannabis testing service providers. One such “ring test,” published in O’Shaughnessy’s journal of medical cannabis in 2011, was conducted by California NORML and included the cannabis labs offering services in the state of California at the time. The results from 10 laboratories were quite revealing and reinforced the fact that the use of certified reference materials is needed in order for lab-to-lab results to be in good agreement. Those labs who “made their own” chemical reference standards rather than purchasing certified ones were found to have results that deviated significantly from the rest of the pack, whereas good agreement was seen across a handful of labs. The test brought to light several basic principles that can act as a guide in evaluating a cannabis lab.
Understanding testing processes and equipment can help guide you in choosing service providers and testing options best suited to your needs. Different service providers specialize in different types of samples and offer different analytical options. In the unregulated environment of cannabis testing at the time of this writing, there is no specified way to perform a particular type of analysis. This leads to a variety of different methods being used, making it confusing for the patient and farmer to interpret and understand how to compare results between service providers. Knowing a few key things can help guide you in the right direction.

This harvest of ‘Bubba Kush’ buds measured from 13 to 19 percent THC. The samples were taken on different parts of the plants.
COMPARISON OF GC AND HPLC OR UPLC
| Gas Chromatography | High-Performance Liquid Chromatography or Ultra-High-Performance Chromatography |
| A low-pressure stream of gas helps move the compounds to the detector | A high-pressure stream of solvent helps move compounds to the detector |
| Detector destroys compounds | Detector usually doesn’t destroy compounds |
| Cannot detect cannabinoid acids | Can detect cannabinoid acids |
| Analysis does NOT produce significant hazardous waste. Eco-friendly | Analysis produces a lot of hazardous solvent waste |
The Potency Testing Process
The basic steps of the potency testing process that affect the accuracy and precision of the results are:
- sampling
- calibration of equipment
- quality of chemical reference standards used
- experience and expertise of the person performing the work
Sampling can be performed at several stages in the movement of cannabis from the farmer to the patient. The product may first be sampled at the farm and provided to a dispensary that then takes a sample to submit to the lab where the cannabis is finally sampled by the lab itself. Each step along the way—as well as the handling of the sample—can influence the final potency results and whether cannabinoids such as CBN (a degradation product) are present. At each point at which it is handled and sampled, the cannabis sample must be representative of the entire quantity of material it is selected from. For the farmer with several pounds of the same strain of cannabis, taking a representative sample means making a selection of buds of various sizes from various plants and various parts of the plant. A recommended sample size to submit to the lab is 0.25 to 1 gram (0.008–0.035 oz) per pound of material.
At the lab, the sample should be cut up or ground to homogenize it or make it uniform. A portion of this homogenized sample is weighed on very sensitive scales and then extracted with solvents such as methanol or isopropyl alcohol for analysis. Scales used to weigh samples should be calibrated regularly, and a dated log of those calibrations should be kept. In some labs, samples are dried to zero moisture content before being weighed for analysis. Depending on how much moisture was in the plant material to begin with, this can greatly affect the results anywhere from 3 to 10 percent by weight. The extraction of the sample involves placing a measured amount of solvent in a vial with the weighed cannabis sample. Different laboratories use different solvents for their extraction. Not every solvent does a good job at completely extracting all the cannabinoids. Methanol, ethanol, and isopropanol are the best options for complete (also known as exhaustive) extraction, whereas acetone and hexane used by some service providers do not extract THC and CBD as completely. This incomplete extraction would cause an overall lowering of the potency results. After the addition of solvent, the extraction process can vary. Some may heat the sample to extract; others may sonicate using ultrasonic waves, or put in a shaker bath or table. Using an ultrasonic water bath is generally the best method for full extraction of cannabinoids. (Find a study of these solvent extraction issues at https://www.restek.com/row).
Depending on the type of sample being analyzed, one way of analyzing it may be more appropriate than another. Most potency and pesticide testing on cannabis samples involves analyzing the sample using some form of chromatography equipment. Two common types are gas chromatography (GC) and high-performance or ultra high-performance liquid chromatography (HPLC or UPLC). These pieces of instrumentation may be equipped with many different types of detectors. Some service providers claim to be superior based on their chosen equipment. Both types of instrumentation yield accurate and viable results when calibrated correctly and used by an experienced operator who can interpret and validate the data output.
Gas Chromatography (GC)
A gas chromatograph is a type of analytical instrumentation in which a low-pressure stream of compressed gas—often hydrogen or helium— pushes a sample from the injector to the detector of the instrument through a small-bore tube known as a column. The column in a gas chromatograph is a 15- to 30-meter length of fused silica tubing coated with various substances on the inside. These coatings and the length of the tubing cause the compounds to separate from one another in space and time as they travel through the column. In this way, samples reach the detector separately and can be measured individually. If an analysis is not performed correctly or is performed too quickly, two compounds may reach the detector at the same time and be measured as one compound, resulting in an erroneous reported result.
Gas chromatographs may be equipped with several types of detectors. The most common types of detectors for cannabis potency testing include FID (flame ionization detector), MS (mass spectrometers) and TCD (thermal conductivity detector). FIDs and MS detectors are the best choices for reliable cannabinoid analysis by GC. Pesticides analysis may include detectors such as ECD (electron capture detector), NPD (nitrogen-phosphorous detector), or PFPD (pulsed flame photometric detector).
In the case of all GC detector types, the sample extract that is injected into the instrument is often heated to temperatures between 302ºF and 392ºF (150°C–200°C). In order to vaporize the sample so that it may be moved from the injector onto the column by the stream of gas, the sample also is combusted when it reaches an FID detector. This sample vaporization will decarboxylate or activate any acidic cannabinoids as if they had been combusted or vaporized. In a mass spectrometer detector, the sample is ionized and fragmented in order to identify the compounds present. Sample vaporization makes gas chromatography the best option for analysis of cannabis samples meant for inhalation by combustion or vaporization. From an analytical perspective, this direct measurement of cannabinoids in their decarboxylated state best mimics the experience of the patient upon inhalation.
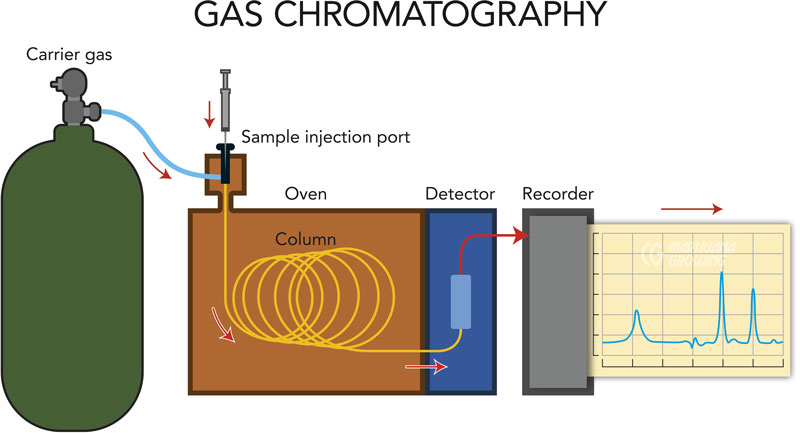
Sample path in a gas chromatograph

Gas chromatograph auto-sampler

High-performance liquid chromatograph. (Ultra-high-performance liquid chromato¬graphs have the same appearance.)
High-Performance and Ultra-High-Performance Liquid Chromatography (HPLC and UPLC)
High-performance and ultra-high-performance liquid chromatography are commonly referred to as HPLC and UPLC. The difference between HPLC and UPLC is the maximum pressure the instrumentation can withstand. UPLC instruments can analyze faster and still maintain accuracy in most cases as compared to HPLC. In HPLC and UPLC a high-pressure stream of solvent helps move compounds from the injector to the detector. As with GC, there are several types of detectors that are commonly used for cannabinoid analysis. UV-Vis (ultraviolet visible spectroscopy) detectors use wavelengths of light passing through the sample to measure what is there. Photodiode array (PDA) and fluorescence detectors use certain wavelengths of light to excite compounds in the detector, which are then measured as they emit photons of light falling back down to a less energetic state. HPLCs and UPLCs may also be equipped with MS detectors. These are generally a second detector in line after the PDA or UV-Vis detectors.
The measurement of cannabinoids in an HPLC or UPLC system can be conducted without vaporizing the sample using heat. As a result, analysis by HPLC or UPLC best mimics the mode of use for edible or ingestible samples. By not heating the sample during sample preparation or analysis, different cannabinoid information can be obtained to understand the properties of the sample as an ingestible form of cannabis. It is important to understand whether or not the cannabinoids are in their acidic form or decarboxylated form for the purposes of ingesting cannabis for therapeutic or recreational uses. THC-acid and CBD-acid have very different psychoactive and therapeutic properties as compared to decarboxylated THC and CBD. For example, THC-acid is nonintoxicating, while heated THC is very intoxicating when orally ingested in very small amounts. For example, one oral dose of THC is 15 mg. It is important to analyze orally ingestible samples by HPLC if you are unsure about whether or not the cannabinoids are decarboxylated and need to verify the content of an ingestible preparation. Many producers of ingestible cannabis preparations do not understand clearly the conditions for complete conversion of cannabinoid acids to THC and CBD, resulting in many cases of partially decarboxylated edibles and tinctures. In some cases it is important to verify that NO decarboxylation has occurred for those patients seeking to use a cannabinoid acid therapy program specifically avoiding the decarboxylated forms.
One of the reasons it is important to restrict the use of HPLC and UPLC to specific samples where it is needed is due to the large amount of environmentally toxic hazardous waste that is produced as a result of performing analysis by HPLC. As described earlier, a high-pressure stream of solvent is used to move analyte from the injector to the detector. This stream of solvent is generally a mixture of acetonitrile and isopropyl alcohol. Its destiny is the hazardous waste bottle, so unnecessary HPLC analyses should be avoided in an effort to have low environmental impact, and use of this method restricted to ingestible samples where it is truly required. Some examples of ingestible samples include tinctures, juiced cannabis, edibles, and capsules.

Cannabis edibles

Two different cannabis buds have distinct cannabinoid profiles.
Comparing Test Results — HPLC vs. GC
A second reason why HPLC and UPLC should not be used on samples meant for inhalation is that the results are not expressed in the form in which the cannabinoids will be consumed when heated and inhaled, the decarboxylated forms THC and CBD, and can give the perception of higher potency than the cannabis contains. For patients on a specific dosage regimen, it is important to be able to accurately calculate THC and CBD consumed. Results from HPLC and UPLC analysis will show THCA, THC, CBDA, and CBD in addition to other cannabinoids such as CBN and CBG. Many laboratories that use HPLC/UPLC for cannabinoid analysis of flowers express their final results as THC=THCA + THC in a sample. This can be confusing to the consumer because the THCA value is not properly mathematically converted to its equivalent amount of decarboxylated THC. THC is the form that will be experienced by the user when smoked or vaporized, thus the potency measure for cannabis intended for inhalation should be in the neutral or THC form rather than THCA because of this.
Expressing results as THC+THCA can cause the expression of “THC” results higher than what the user will experience with smoking or vaporizing because of the following considerations: THCA and CBDA are heavier molecules than THC and CBD. To express the total decarboxylated THC that a sample may have in it, you must first adjust the THCA value by multiplying THCA or CBDA by a factor of 0.88 to account for this difference in molecular weight. Understanding the conversions in the figure below can help you compare results that were generated by HPLC/UPLC and GC. You should also consider whether or not the results are expressed on a dry-weight basis as well. You should know how the lab you are using calculates and reports results so you know if you have to perform any of the conversions noted below to make the results meaningful for the way you’ll be using the cannabis.
To convert THCA to THC:
- Multiply the THCA value by 0.88%
- Example: 21% THCA × 0.88 = 18.5% THC
To convert THC to THCA:
- Multiply the THCA value by 1.14
- Ejemplo: 17% THC × 1,14 = 19,4% THCA.
The same applies to CBDA and CBD
As you can see, depending on your sample type (flower, concentrate, edible, or tincture), one type of equipment may provide more meaningful results, depending on your use of cannabis.s.
- Cannabis for inhalation samples such as flowers and concentrates to be smoked or vaporized should be analyzed by GC.
- Cannabis for oral ingestion should be analyzed by HPLC or UPLC if state of decarboxylation is unknown or unverified.
- When evaluating test results, you should know if they are expressed in terms of:
- Acid forms or decarboxylated forms.
- Dry weight or full moisture.
Many patients and farmers wonder what results are typical and how the information varies by strain:
| Potency Rating | THC Potency (% by weight) |
| mild | 3—10 |
| moderate | 10—16 |
| strong | 17—20 |
| very strong | 21+ |
Typical THC results for cannabis flowers
- Ranges from mild to very strong
- Average: 16.5 percent
| Potency Rating | CBD Potency (% by weight) |
| low | 0—2 |
| moderate | 3—5 |
| high | 6—20 |
Typical CBD results for cannabis flowers
Often not found in significant amounts
Always accompanied by THC
CBD-rich: >4% CBD by weight
Alternate Methods of Potency Testing: Thin-Layer Chromatography (TLC)
Thin-layer chromatography tests measure the cannabinoid profile for up to 6 cannabinoids: THC, THCV, CBD, CBN, CBG, CBC, and their corresponding acids. The tests cost about $10 USD each and take some practice to perform with accuracy. The sample is prepared and mixed with a solvent. A selective dye detects the cannabinoid levels and shows them on blotter paper. The results are then interpreted and compared to a chart. Every cannabinoid reacts differently with the dye, resulting in distinctive colors. Please see www.alpha-cat.org for more information on thin-layer chromatography.
A segment of service providers presents results from TLC (thin-layer chromatography) on what are often referred to as “test strips.” In some cases, these test strips are promoted as being able to provide accurate potency results for cannabis. In general, without specialized equipment, test strips are only viable for use to tell whether or not certain cannabinoids are present, but not to tell how much is present (i.e., potency).
How Patients Can Use Potency Results
Patients are often faced with a dizzy ing array of options in a medical cannabis dispensary. Having accurate cannabinoid potency information can help patients select the best medicinal cannabis for their ailment or condition. Knowing a bit about the different effects and medical applications of various cannabinoids can help the patient have a successful and efficacious experience. THC is the main active ingredient in cannabis, responsible for some of the intoxicating effects of cannabis. THC is often used to treat pain, nausea, insomnia, and loss of appetite—among many other ailments. CBD is the second most predominant cannabinoid and is considered a nonintoxicating ingredient in cannabis, although when consumed there are clear mood-altering effects for most. CBD provides relief from muscle spasms, seizures, and anxiety, is known to have antibacterial and anti-tumor activity, and prolongs the effects of THC. CBN is the degradation product of THC. The presence of CBN can indicate that a product is old or has not been stored properly. CBN forms when THC is exposed to UV light and oxygen over time. CBN does have some therapeutic properties and has been shown to lower heart rate and reduce convulsions. Cannabinoid acids such as THCA and CBDA have been reported by patients to provide relief from symptoms of spasticity and convulsions.

Medical cannabis dispensaries like the Abatin Wellness Center in Sacramento, California, are well stocked.

Test the cannabinoid profile of vegetative plants to see if they are candidates for a breeding program.
Cannabinoid Potency Testing as a Tool for the Farmer and Breeder
As the number of medical cannabis users remains on the rise, there is an ever-increasing demand for options with specific THC:CBD ratios. Likewise is the increasing demand for cannabis with the highest potency levels, since potency readily translates to the amount of active compounds and the value of the cannabis in the current marketplace. The demand for cannabis with specific THC:CBD ratios is often driven by a population with a specific ailment or disease. One such example gaining notoriety at the moment concerns the specific needs of children with a type of epilepsy known as Dravet syndrome. Reports of 400 to 800 seizures per day have not been uncommon for these children, who also suffer from developmental, cognitive, and emotional issues. Desperation leads parents of these children to pursue experimentation with cannabis extracts as a treatment for their child’s condition. The type of cannabis required by these children to reduce their seizures is almost entirely CBD with very little THC. The preferred THC:CBD ratio for most Dravet children being medicated with cannabis is 1:20 or greater. This type of cannabis was first isolated and identified only about 4 years prior to publication of this book. At that time there was only one known plant and source. The miracle that this particular type of cannabis represented for the families of these children has pushed CBD-rich cannabis toward the mainstream, as illustrated in a CNN documentary by Dr. Sanjay Gupta. As a result, the demand for cannabis with this specific THC:CBD ratio spiked.
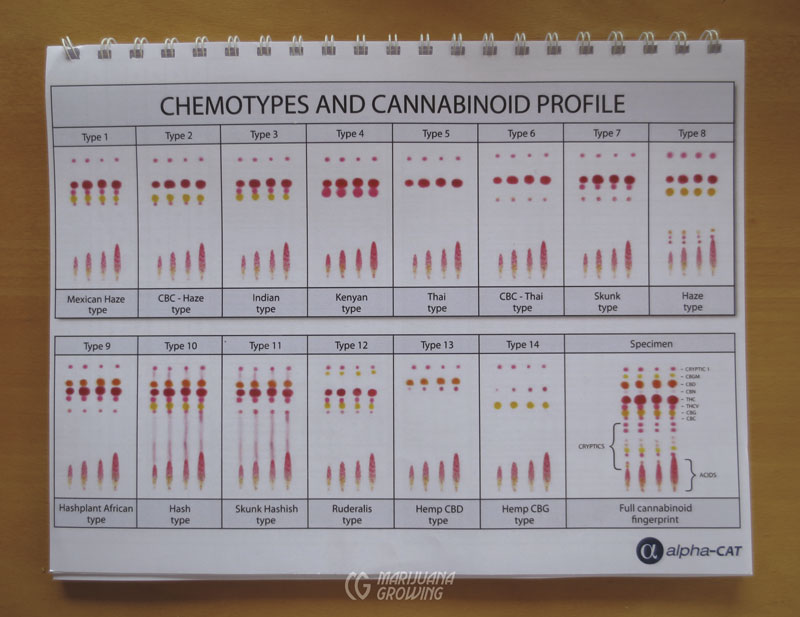
The cannabinoid profiles of 14 different varieties of cannabis are represented in this page of thin-layer chromatography tests.
Just as that first 1:20 strain (known as ACDC) was first isolated, a technique was developed in an independent cannabis lab in California based on preliminary research conducted by Pacifico et al. in Italy that would allow the rapid development and isolation of this rare and now important type of cannabis. The program was developed at Pure Analytics for analyzing and interpreting vegetative-stage cannabinoid data to facilitate rapid high-CBD isolation, ratio characterization, and CBD and THC potency optimization, as well as the prediction of absolute potency numbers when plants are sampled within the correct stage of development. In the 2014 growing season alone, hundreds of 1:20 THC:CBD phenotypes were isolated that were to be grown into plants and made available on the medical cannabis market. This technique, when it is performed properly and the data is interpreted correctly, can allow farmers to have the crystal ball they always wanted, to see what the flowers will produce before they are formed.
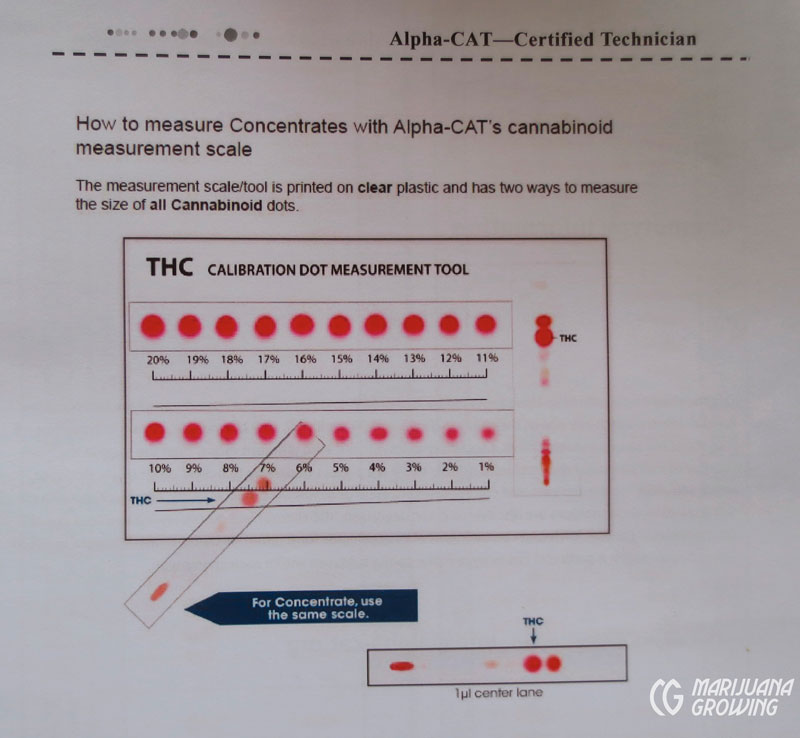
Once prepared and tested, the sample circles are measured with a plastic calibration measurement tool to discern cannabinoid levels.
With a vegetative-stage cannabinoid test performed using the proprietary protocol developed by Pure Analytics Lab in California a farmer may identify his THC:CBD ratios early in the growth cycle, identify plants with the highest cannabinoid accumulation potential, and obtain a prediction of final flower potency. The farmer may determine cannabinoid ratio in both males and females, and these ratios will remain in place through the full maturation and flowering process of the plant. Meaning, a 1:20 cannabinoid ratio identified in a two-week-old vegetative plant will result in flowers with the same ratio of THC:CBD. This technique also allows identification of the seedlings with the greatest cannabinoid accumulation potential, enabling the farmer to identify his most potent strains with desired THC:CBD ratios while still in the vegetative stage. This is a powerful advancement allowing cloning of both females and males with desired traits in the vegetative stage to enable the strategic mass breeding of genetically identical clones, thus creating a more consistent resulting seed population. For more information on using vegetative-stage testing, visit www.pureanalytics.net/blog.

Heavy-yielding, CBD-rich ‘Cannatonic × Sour Tsunami’ was developed using tests provided by Pure Analytics.

Measure the cannabinoid content of male plants before they bloom. Choose males with desirable cannabinoid profiles for breeding programs.
Cannabis Safety Testing — Pesticides, Microbiological and Other Contaminants
While the testing of cannabinoids is endlessly fascinating, other types of cannabis testing are also meaningful to the health and welfare of patients using cannabis that they have not controlled the production of. Many medical cannabis dispensaries test for various types of contaminants that may be harmful to patients. These include pesticides analysis and microbiological analysis, as well as tests for chemical residues such as solvent, solvent impurities, heavy metals, and nutrient residues. There are many different ways to perform a given type of analysis. For example, a pesticides analysis may be performed by a variety of different pieces of equipment.
For pesticides it is important that a chromatographic analysis is performed every time, and that the analysis is sensitive enough to be meaningful. There are “shortcuts” that are tempting to use because they are cheaper and faster, but they are not an adequate replacement for a full chromatographic analysis. The problem with shortcuts such as immunoassay screens or general screens using a mass spectrometer for detection is that they generally cannot achieve a detection limit that is truly meaningful relative to potential pesticide contamination. For example, if a contaminant can pose a health risk at 50 parts per billion or ug/kg, then a pesticide screen that can only detect contamination at higher levels such as 100 parts per million or mg/kg is not meaningful or particularly useful.
When comparing different approaches to pesticide analysis, you need to consider a few key areas:
- Equipment used.
- Sensitivity of the method, also referred to as “detection limit”.
- Compounds analyzed for and reported on.
- Whether third-party-certified reference standards are used when identifying a positive hit.
It is important to realize that not every type of equipment listed can see every pesticide that may have been used. Some detectors are very specific and some are more general. It follows that the more generalized the detector is, the more reduced its sensitivity to potential pesticide contamination will be. As noted earlier, unless specially configured, a mass spectrometer is not the best choice for pesticide-contamination detection due to its higher threshold of detection, meaning it likely can’t see trace-level contamination. The use of mass spectrometer detection may also lead to bad habits in the lab. Pesticides analysis is not trivial, nor is it cheap to perform. To be done properly each class of pesticides should be analyzed and evaluated separately using the detector most sensitive to that class of compounds. For example, chlorinated pesticides should always be confirmed by GC-ECD (gas chromatography with electron capture detection) and phenoxyacid herbicides by HPLC-FD (high-performance liquid chromatography with fluorescence detection). They should also always be confirmed against certified reference material of the pesticide. Meaning, if myclobutanil is reported as present, myclobutanil purchased as a third-party-certified reference standard from a chemical company should have been analyzed along with the sample. If this was not done, the result was not validated. That leads us to an explanation of how the use of mass spectrometers can lead to bad habits in the lab.
TYPES OF INSTRUMENTATION SUITABLE FOR PESTICIDES ANALYSIS
| Acronym for Equipment | Description |
| GC-FID | gas chromatography with flame ionization detection |
| GC-ECD | gas chromatography with electron capture detection |
| GC-MS | gas chromatography with mass spectrometer detection |
| GC-PFPD | gas chromatography with pulsed flame photometric detection |
| GC-NPD | gas chromatography with nitrogen phosphorous detection |
| HPLC o UPLC-UV-Vis | high- or ultra-high-performance liquid chromatography with UV-visual photometric detection |
| HPLC o UPLC-MS | high- or ultra-high-performance liquid chromatography with mass spectrometer detection |
| HPLC o UPLC-PDA | high- or ultra-high-performance liquid chromatography with photodiode array detection |
| HPLC o UPLC-FD | high- or ultra-high-performance liquid chromatography with fluorescence detection |
Mass spectrometers are amazing pieces of equipment with immense capability to identify unknown compounds that may be in a mixture. Mass specs are equipped with access through their software to a powerful NIST (National Institute of Standards and Technology) database developed over the years by thousands of scientists who have submitted their data to the NIST. It allows one to run a search of the database relative to a datapoint from a mass spec analysis. The software searches the database and finds the closest match with a probability rating that it is a true result. This is acceptable for a preliminary identification of a contaminant but not a basis for reporting that the compound is present. It must be verified against the certified chemical standard for the same compound in order to be a legitimate result identifying a specific compound.
Here are some key questions to ask a service provider when learning about their pesticides analysis:
1. What type of equipment do you use to perform a pesticides analysis?
- You want to hear “chromatograph” in the answer. At a minimum, all samples need a chromatography screen from one type of equipment listed on here) . Note that not all equipment has the same sensitivity or can “see” the same compounds..
2. Which compounds do you analyze for?
- They should be able to provide you a list of compounds they can detect. If a compound is not on their list, they won’t “see” it in the analysis.
- If the response is, “We test for everything,” this may mean they are not using certified reference standards. This is the response that those performing a mass spec screen often provide. Not only are reference standards probably not being used, but it is unlikely that the detection limit is meaningful.
3. What is your detection limit?
- You want to hear “parts per billion (ppb) range to low part per million (ppm) range.” Detection may vary depending on which chemical it is.
- This may also appear as μg/kg range to low mg/kg range.
Microbiological analyses are also used to screen medical cannabis. The majority of service providers screen for mold and fungus contamination while others also screen for bacterial contamination, which becomes more meaningful relative to products intended for oral ingestion. Methods used to screen for microbiological contamination include microscopy, PCR analysis (polymerase chain reaction), plating and culturing with plates that can indicate classes of organisms, mycotoxin screens by TLC, and more. All but microscopy may provide false positives due to mishandling or cross-contamination of samples.
Analyses for heavy metals are usually performed using an ICP-MS (inductively coupled plasma mass spectrometer) and if done properly by defined methods offer a fairly reliable analysis. Nutrient residues may be analyzed by a variety of methods depending on what type of contamination is anticipated. ICP-MS and ion chromatography would be the two most likely approaches.
Focus on Safety — Aspergillus Contamination, Why It Matters
Aspergillus is a genus that consists of more than a few hundred mold species. It is found in most climates where cannabis grows. Some Aspergillus species can cause grave illness in animals— including humans. Aspergillus fumigatus and A. flavus are the most problematic because they produce aflatoxin, a toxin and carcinogen. Both species can contaminate cannabis—including edibles. A. fumigatus and A. clavatus species commonly cause allergic diseases. Other Aspergillus species frequently infect grain crops.

Growing organic medical cannabis is one of the best ways to avoid contaminated medicine.
Pulmonary aspergillosis is usually caused by A. fumigatus, which results in paranasal sinus infection. The symptoms include cough, chest pain, fever, and breathlessness; these common symptoms make diagnosis difficult. Patients with asthma, cystic fibrosis, sinusitis, AIDS, weak immune systems, and weak lungs are susceptible, as are chemotherapy patients.

