Casual and serious cannabis breeders working with Mother Nature use selective breeding have transformed a wild plant into countless varieties of medicinal cannabis. New varieties are easy, fun, and satisfying to create. Home cannabis gardeners breed plants for many reasons—to increase yield, cannabinoid profile, disease and pest resistance, and so forth. Most often, modern medicinal cannabis has been bred for high cannabinoid content (specifically THC), for heavy yield, for early harvest indoors, and occasionally for harsh outdoor climates. Today, more attention is being given to other cannabinoids, such as CBD, and cultivation stress—resistance to diseases and pests and tolerance of drought and cold.

Nevil, founder of the Seed Bank of Holland, traveled the world to find the best cannabis seeds.
Countless cannabis gardeners are patiently applying their imagination and creativity using basic breeding (sexual propagation) techniques to create new generations of seed. Breeding is easy, low-cost, fun, and exciting. It requires minimal time and skill but results in big rewards.
The basics outlined in this chapter show any gardener how to start breeding and create new generations of viable medicinal cannabis seeds. Indoor, backyard, and greenhouse breeding is essential to acclimate varieties to local climates and to increase resistance to diseases, pests, and stress. Indoor and greenhouse cultivation can expedite outdoor breeding programs. Unfortunately, medical cannabis seeds are not available in some jurisdictions, and indoor breeding is a matter of necessity for patients and caregivers.

This colorful ‘Pakistan Chitral Kush’ from CannaBioGen is a pure landrace line from Chitral, Pakistan. It is also on the cover of this book!

Seated in the center in white, Sensi Seeds owner Ben Dronkers is pictured collecting seeds in Afghanistan.

The Internet has changed the availability of genetics (seeds) worldwide.
On the surface, breeding is simple: pollen from a male plant is used to fertilize (pollinate) a female plant, and seeds result. In fact, this is what most cannabis crosses are based upon—crossing a cannabinoid-potent male with a cannabinoid-potent female plant, often referred to as “pollen chucking.” However, a few important genetic details are involved.
Crossing cannabis plants can become very complex, and plants take time to grow to express their genes. Modern plant breeders study statistics, chemistry, plant physiology, microbiology, and other disciplines to understand and breed plants. The basic information presented here is in an easy-to-understand format so it is as straightforward to reference as possible. Cannabis breeding is a long-term process, and the possible outcomes of progeny are infinite.

To date, much of the cannabis breeding in the USA has been done by clandestine breeders such as Mel Frank, who introduced varieties such as ‘Afghani’, ‘Nepalese’, and ‘Mexican’, as well as many Colombian varieties.
Much more scientific information about plant breeding is available on the Internet, including modern genetic principles by Gregor Mendel and inspiring stories from American breeders such as Luther Burbank. Be sure to read about Dr. Kevin McKernan, who mapped the cannabis genome!
Please continue studying after you have digested this basic chapter. Remember, breeding cannabis is a long-term endeavor—even in an indoor garden room where four generations are possible.
The cannabis world today consists of millions of small gardeners in more than 200 countries around the world*. Many gardens exist in unstable and difficult growing conditions. Adopting new cannabis varieties developed for cultivation in faraway climates and indoor grow rooms does not meet the needs of many outdoor gardeners and their local climates. The Internet* and cannabis gardeners’ ingenuity have broken ground for gardeners everywhere to participate in the breeding process. Breeding goals can now be identified and defined by local gardeners instead of large seed companies or fad-driven advertising.

The ‘Afghani’ × ‘Congolese’ plants in the foreground are the product of selective breeding. The miscellaneous sativa plants in the background represent wild cannabis plants. (MF)

Choosing the proper male breeding stock is half of the equation. Choose the best males possible that exhibit the desirable traits you want.
For example, the following YouTube video has received more than 5 million hits from more than 180 countries: www.youtube.com/user/jorgecervantesmj.
Most gardeners prefer to acquire seeds from a reputable seed company, and too often assume these seeds are consistently of the highest genetic quality. Often, commercially available seeds are produced by a few large seed makers who wholesale to resellers, many of them with Internet sites and retail store outlets. Other seeds are produced by basement growers, small startups, or small- to medium-sized established companies.
Few small companies can maintain a library of true-breeding stock and breed stable parent stock over time. Stock produced by large companies usually fills demand and maintains standards.

This beautiful garden of ‘Sonoma Coma’ females is the result of years of selective breeding in a valley full of vineyards in California.

Individual male flowers that appear on a female flower bud are often called “bananas” because they look like little bananas.

This ‘African’ grown in 1977 has a small male flower on this predominantly female flower bud. This is an intersex male flower.
Unforgettable Qualities of Cannabis
Cannabis has a unique set of qualities that influence its life cycle and reproduction. Working with these natural qualities is essential to breeding cannabis. Please remember them when working on all breeding projects.
Cannabis is photoperiodic reactive, flowering under 12 hours of uninterrupted darkness and 12 hours of light, which gives breeders the ability to grow up to 6 crops every year. These characteristics facilitate fast-track breeding because 6 years of crosses can be completed in 1 year.
Cannabis is dioecious; it produces each of the male (staminate; stamen-bearing) and female (pistillate; pistil-bearing) sexual organs on different individuals, and it is one of the few annual dioecious plants. This quality makes it very easy to cross an individual male cannabis plant with an individual or population of females.
Male (staminate) and female (pistillate) plants are easy to distinguish. See close-up details of male and female flowers along with full descriptions below in this chapter.
Monoecious varieties produce both staminate and pistillate flowers on the same plant. Monoecious varieties are mainly used for hemp seed production. Monoecious varieties do not make good medical cannabis breeding stock. Plants with both male and female flowers are often called by the misnomer “hermaphrodite.”
Outcrossing plant: Outcrossing cannabis grows best when plants cross with other plants with different genes. Corn, dogs, cannabis, and people are all outcrossers. Backcrossing and inbreeding cannabis for too many generations will be detrimental.
Intersexuality occurs when flowers of a male plant grow on a predominantly female plant, or when female flowers grow on a predominantly male plant. It is a trait that can be caused by both genetic and environmental factors. Intersex plants with the inherited gene grow flowers from both sexes on the same plant even in perfect growing conditions. Intersex plants are often called by the misnomer “hermaphrodite.”
Indoors, plants are easily exposed to stress—inconsistent or extreme temperature, light cycles, fertilizer, pH, etc.—and this stress can cause female plants to grow an occasional male flower. Breeders prefer not to perpetuate the intersex gene, and when possible, they eliminate it. A single male flower on a female plant can pollinate a big part of the female. See “Feminized Seeds,” on page 521.
Physiology of Male and Female Flowers
Male Flowers
Male (staminate) cannabis flowers are about 0.25 to 0.5 inches (0.6–1.3 cm) long, and thousands of individual flowers can develop on a large plant. Most of the flowers develop in loose clusters (cymes or cymose panicles) of about 5 or 10 flowers each, borne on tiny branches and their side (lateral) branches. The clusters can pile atop each other to form dense aggregates of hundreds of individual flowers, particularly at the ends of stems and branches.
Each male flower’s calyx consists of 5 tepals (sometimes identified as “sepals”)—usually white, yellowish, or greenish, but often tinged purple—that might be described as “petals,” and 5 pendulous stamens that bear pollen in sacks called anthers. Anthers hang by a thin, threadlike filament, and together, filament and anther make up the stamen. Once mature, 2 openings on opposite sides of each anther open zipper-like, starting at their base, to slowly release their pollen into the wind, carrying it (hopefully) to stigmas. It has been estimated that the thousands of flowers on a single male can release more than 500 million pollen grains.
Unopened male flower clusters remind some growers of tiny grape clusters, and fresh anthers look somewhat like bunches of tiny bananas. Male flowers are simply called male flowers or male flower clusters, and the pollen holders are referred to as either stamens or anthers.
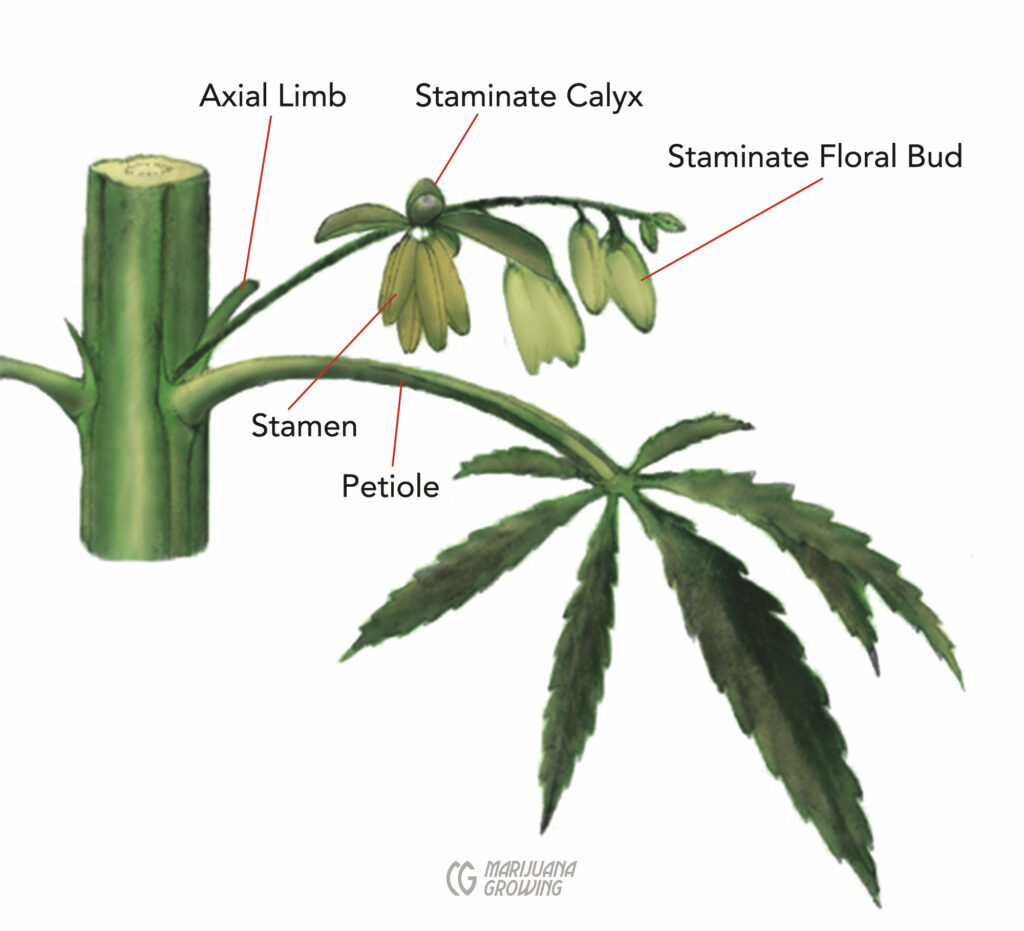
This drawing shows the main parts of a male cannabis plant.

‘Jack Herer’ male (staminate) flowers fully formed in clusters but not yet open. (MF)

Outer tepals on the ‘Skunk #1’ staminate calyxes have separated, exposing the pollen-containing anthers. (MF)

As anthers on this ‘Jack Herer’ mature, they rupture to disperse superfine grains of pollen into the air. (MF)
Female Flowers
Each female marijuana flower has 2 stigmas that protrude from the single ovule enclosed in the bracts; fresh stigmas are “fuzzy” (hirsute), about 0.25 to 0.5 inches (0.6–1.3 cm) long, and usually white, but sometimes they are yellowish or pink to red, and, rarely, lavender to purple. Stigmas (stigmata is another botanical plural) are the pollen catchers. Stigmas are often misidentified as pistils. By definition, a pistil is all of the reproductive parts of a flower—2 stigmas and an ovule make up the female pistil. Each flower then has only 1 pistil but has 2 stigmas. The term is misused in much of popular culture, which describes a single cannabis flower as having 2 pistils.

Staminate anthers on this ‘Skunk #1’ grown in 1987 continue to split open and disperse more and more pollen into the air. (MF)

Anthers hang in the wind after dispersing all their pollen throughout several days. (MF)

Pollen from this ‘Skunk #1’ has been dispersed from anthers in the foreground. Staminate calyxes in the background will disperse pollen in the near future. (MF)

Compare this tiny male flower to the size of a US penny. (MF)
Stigmas begin dying after pollination and begin to turn rust-colored about 3 days later; if not pollinated, as with sinsemilla (seedless buds), stigmas begin to die when they are about 4 or 5 weeks old. Upon landing on a stigma, a pollen grain germinates and begins to grow a pollen tube through the style passageway to pass its DNA to join the ovule’s DNA (the 2 stigmas of each female flower make up a bifid style). The fertilized ovule becomes a fruit, essentially a single seed (an achene). The perianth, which includes the calyx, tightly clasps the seed and often contains tannins, which give mature seeds their mottled or spotted coat. Between a thumb and finger you can rub the perianth off of seeds. A well-pollinated single bud develops dozens of seeds, a cola easily holds many hundreds, and even a small, but thoroughly pollinated female can bear thousands of seeds.
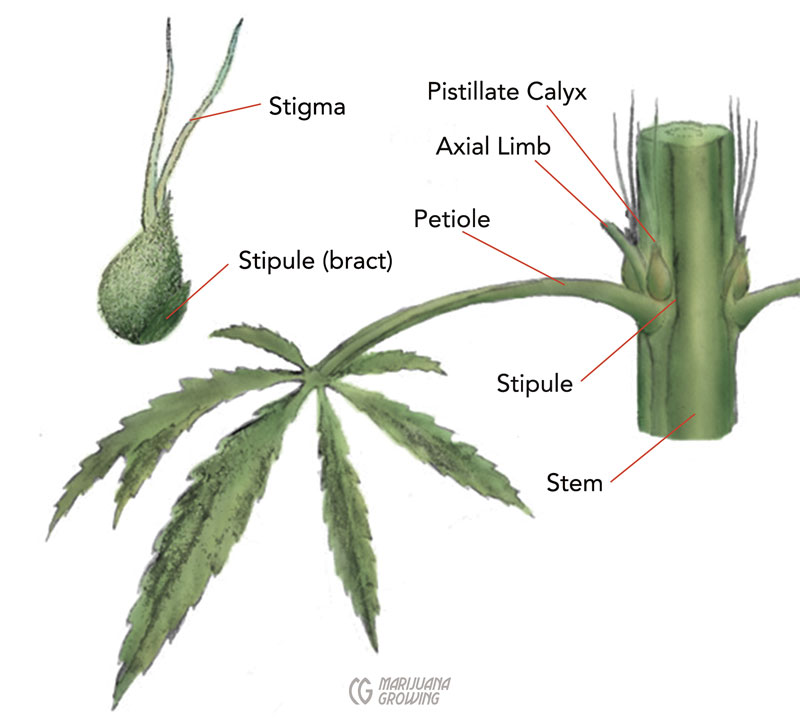
This drawing shows the main parts of a female cannabis plant.
Each female flower has a single ovule partially enclosed in its perianth, which is encapsulated by bracteoles, which are covered by a whorl of bracts. The bracts and bracteoles are small, modified leaves that enclose and protect the seed in what some growers refer to as the seedpod.
Bracts contain the highest concentration of THC and other cannabinoids of any plant part and about 50 percent of a plant’s total THC. The perianth and its calyx contain no THC.
By definition, a perianth consists of a corolla and a calyx. In more familiar showy flowers, the corolla is the brightly colored petals we generally appreciate when looking at flowers, and the calyx is the smaller green cup (sepals) at the flower’s base. Bright showy colors, large flower sizes, and enticing fragrances have naturally evolved to attract insects such as bees and flies, or animals such as birds and bats that collect and transfer pollen (unintentionally) to other flowers. Cannabis flowers are not brightly colored, large, or enticingly fragrant (at least to most nonhumans); cannabis plants are wind-pollinated with no need to attract insects or animals to carry males’ pollen to female flowers; cannabis plant parts never naturally evolved into colorful, attractive, or showy parts. Cannabis breeders, though, do breed for fragrance and color once cannabinoid content is firmly established.

The seed bract still covers the perianth, pistillate calyx, gametes, and ovule connected to a pair of stigmas. (MF)

The seed bract has been stripped away to reveal the perianth and pistillate calyx, both of which appear transparent, covering the gametes and ovule. Note the white stigmas have not been pollinated. (MF)

Stigmas start dying back as soon as pollen is ushered down the shaft to unite with the ovule below. (MF)
The cannabis perianth is only about 6 cells thick, so to distinguish calyx cells from corolla cells is best left to botanists. This book uses the botanically correct term bracts for the green or purple, resin-gland-studded specialized “leaves” encasing each female flower, and uses perianth or calyx for the translucent “veil” that clasps and covers about 60 to 90 percent of a mature seed.
Hopefully, when growers use botanical terms such as calyx, bracts, stigma, pistil, anther and stamen, they will follow this book and use the terms correctly. Since readers will find seed catalogs and Internet sites calling bracts calyxes and stigmas pistils, it is important for readers to understand this confusion when reading other sources. Hopefully, this chapter will help get us all on the same page.

The stigmas on this ‘Haze’ × ‘Northern Lights’ × ‘Sensi Star’ flower top are just starting to turn a rusty color. (MF)

The perianth on this ‘Skunk #1’ can be seen near the top of the seed on the left. (MF)

The female perianth is clearly visible as a nearly transparent layer covering the seed. (MF)
Sexual Propagation
Sexual propagation is the process in which male and female sex cells (gametes) from separate parents unite in the female plant to form what will eventually mature into a new, genetically distinct individual. This process occurs when pollen from a male (staminate) parent unites with an ovule within the ovary of a female flower to create an embryo. This embryo, when mature and fully developed, will become a seed.
In nature, cannabis is wind-pollinated. Male flowers shed pollen (dehiscence), dispersing millions of grains into the wind. Wind carries the pollen to a “chance” rendezvous and acceptance by a female stigma.
Pollination occurs when male pollen grains land on a female stigma. The evolutionary attraction is both physical and chemical. The grain of pollen, with moisture found in the stigma, germinates. This is the best part: A grain of pollen germinates just like a seed, sending a taproot down, but instead of sending it into the ground, the grain of pollen sends the “root” down the stigma toward the ovary. Once united with the ovary, the pollen fertilizes the ovule. This union creates an embryo that grows within a seed coat. When mature in 4 to 6 weeks, the seed can be planted.
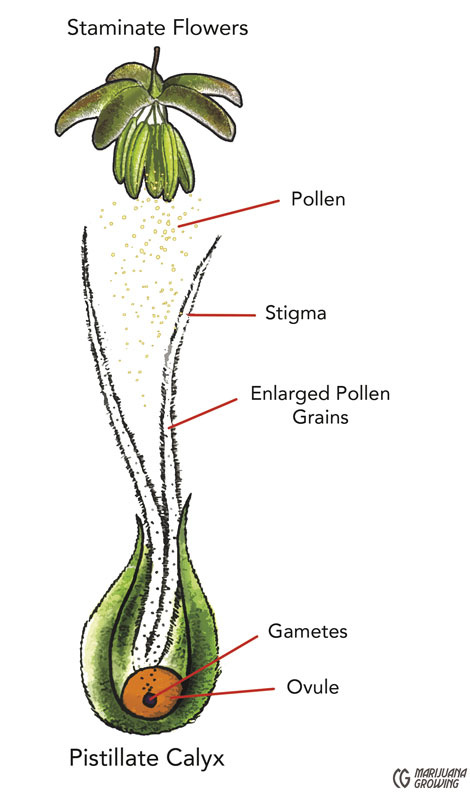
Fertilization occurs when the tiny grain of male pollen sticks to the stigma. Then it develops a tube through the style and releases 2 gametes, 1 to fertilize the ovule and 1 to fertilize the endosperm (double fertilization). Seeds are the result of this sexual propagation and contain genetic characteristics of both parents. Once fertilized with male pollen, female plants put the bulk of their energy into producing strong, viable seeds.
Actual fertilization takes place when the minute grain of male pollen sticks to the stigma. The successful angiosperm pollen grain (gametophyte) containing the male gametes (sperm) gets transported to the stigma, where it germinates and its pollen tube grows down the style to the ovary. Its 2 gametes travel down the tube to where the gametophyte(s) containing the female gametes are held within the carpel. One nucleus fuses with the polar bodies to produce the endosperm tissues, and the other with the ovum to produce the embryo hence the term double fertilization.

Close-up photo of a female stigma shows that no resin glands are located on the entire length. The well-defined protruding growth appears as fuzz on the stalk of the stigma. The stigma is the plant version of a vagina. It is covered with stigmatic fluid that acts like glue when a piece of pollen lands on it. The fluid is packed with sugars that are food for the pollen. When in place, the grains of pollen start growing a new “pollen tube,” a long tunnel that pushes through the tissue of the style all the way to the ova, where it fuses with egg cells to create a little baby plant. The ovules go through a series of steps known as meiosis, a type of cell division by which a cell duplicates into 2 genetically identical daughter cells. The chromosomes in the cell nucleus separate into 2 matching sets of chromosomes, each with a nucleus.
Deoxyribonucleic acid (DNA) or “genetic material”* is coiled into long strands or chromosomes. The DNA is located inside the nucleus of each cell. When cannabis is pollinated, each individual seed inherits 10 different chromosomes from the male, and 10 different chromosomes from the seed mother—20 chromosomes total. Each seed has 2 copies of each of the 10 chromosomes, or 1 full genome each. There are 2 copies of every gene in the plant, 1 from the mother and 1 from the father. Every cell in the plant has a copy of this unique DNA. The genetic code of this unique individual is embedded in a specific location along the length of the chromosome strands.
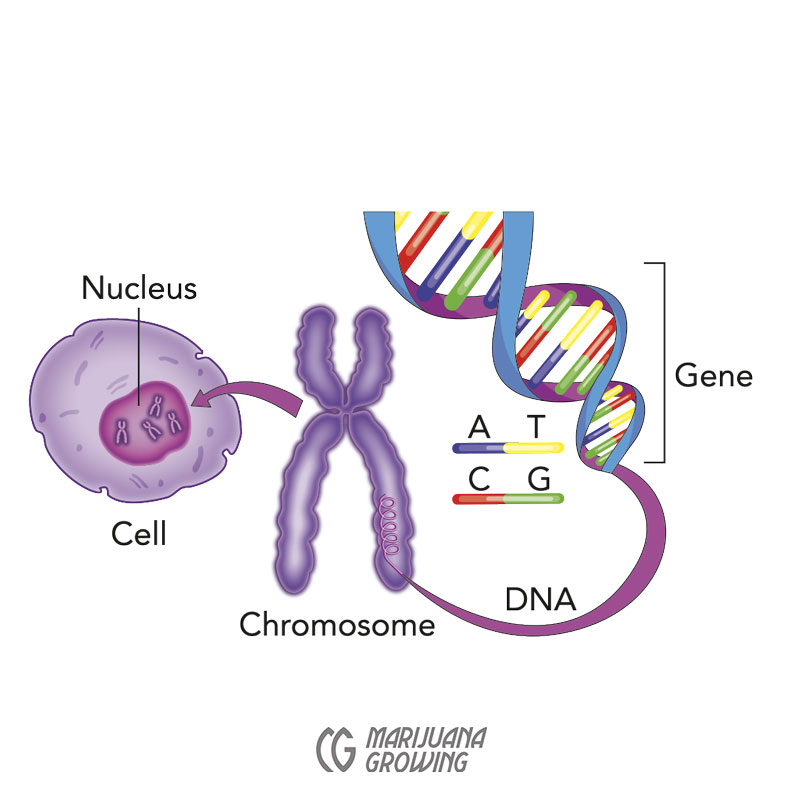
Each seed contains genes from both parents. Progeny grown from seed usually have slightly different traits than other plants from the same seed lot. The same happens in humans; biological children are different from one another in many aspects and at the same time they resemble their parents. In cannabis, the variability is marked, like it is in apples.
Sexual reproduction is used to cross different individuals with a population or family of plants. It can also be used to hybridize unrelated lines and inbreed their offspring. This phenomenon, “recombination of traits,” also gives breeders the opportunity to recover individuals with a combination of the positive traits of both parental lines.
Genes are hereditary units that consist of a sequence of DNA that resides in a precise place on a chromosome and it determines a specific trait in cannabis. Little bits of DNA are codes or templates for proteins.
Proteins are made in the sequence of DNA. Like instructions in a recipe for making brownies, the DNA and sequence of proteins is the recipe or instructions.
Having 2 versions of the same protein, from 2 different genes, is better than having just 1, particularly if the protein plays some vital part in cannabinoid production. This effect is called overdominance. For example, if there are 2 different proteins, and both work well but 1 works a little better under hot conditions and the other works well under cool conditions. Having 2 versions of the same protein gives the plant a wider range of climates where it produces effectively. See “Multiline,” on page 531.
The majority of DNA is the same; it deals with basic cell processes, photosynthesis, chlorophyll production, etc. A few or a combination of genes control variables such as height, leaf shape, fragrance, and disease resistance. But we are not sure exactly which genes are responsible for specific traits, even though the cannabis genome has been mapped. These traits are influenced by multigene families (a group of genes that evolved to become a little bit different from each other, even though they started out as copies of the same gene). Knowing the named genes would make it easier to find individual plants with your desired traits. But single genes controlling specific traits of cannabis are neither isolated nor well studied.
Multigene traits allow you to fine-tune to your favorite characteristics. For example, a single gene that controls leaf size would give only 2 leaf sizes, large and small. Many genes influencing the same trait provide many different leaf sizes.
Naturally occurring mutated cannabis genes are uncommon. They are abnormal genes that are mutations of normal genes. When a mutated gene combines with a normal gene, there is no detrimental outcome. But when 2 mutated genes join, the result is much different.
For example, in people and animals the number of albinos or dwarfs is minimal. The same is true in cannabis. Growing large populations of cannabis or treating cannabis with stress or chemicals will bring about mutation. Overall, most cannabis plants grow normally with no mutations whatsoever. Many different genes control the desirable traits we care about. Broken recessive genes do not play a role in most breeding programs.
Deleterious recessive genes: The plants most likely to have the same dangerous mutation in the immediate family are inbred. Marry your sister and inbreeding genes take over to start all kinds of problems because recessive deleterious genes appear.

‘Royal Moby’, predominantly sativa, is very THC-potent. This variety is quite similar to ‘Moby Dick’ from Dinafem Seeds in Spain. Once a good variety reaches the marketplace, many similar varieties appear within a year or two.
Classical Cannabis Breeding
Classical breeding is an ancient cyclical process that cannabis breeders still use today. Decisions are made based on observation of large numbers of plants; the breeder does not know exactly what genes have been introduced to the new cultivars. All the breeder can do is choose plants based upon visual inspection, smell, and gut-feeling.
Classical cannabis breeding is simple: 2 varieties, a male and a female, are chosen. Each parent has desirable characteristics—fragrance, potency, mold resistance, and so forth. The male pollen fertilizes the female flower and their genes combine into a new genetic mix contained in the seed.
The next step is to choose individual plants with the desirable traits of both parents. A lot of the time you get lucky and the offspring carry the desirable genes and traits. Cannabis breeders often take clones of these desirable individual plants. Too often they do not retain the male plant and have a “clone only” variety.
When a desirable trait has been bred into a plant, crossing other plants to this parent makes new plants similar to the favored parent. For example, to make the mildew-resistant progeny of the cross most like the high-yielding parent, the progeny will be backcrossed to that parent for several generations (see “Backcrossing,” page 519). This process removes most of the genetic contribution of the mildew-resistant parent.
To breed for mold resistance, grow out plants in moldy conditions. Remove plants from garden that contract mold easily. Keep plants that do not get mold or are late to get mold. Breed plants that do not mold.
It is very difficult to isolate specific genes to guarantee specific qualities such as extreme resistance to powdery mildew or insect and mite attacks. There are recessive genes and dominant genes that are controlled by alleles; this is where breeding becomes much more complex. See “Influence of Alleles.”
Other traits, such as acclimatization to a specific climate, are relatively easy because the plants that grow best in the environment are continually selected. Organic gardeners breed plants acclimated to their outdoor climate and achieve much higher yields. For this reason, organic medical gardeners in Northern California are able to grow 10-pound (4.5 kg) plants.
To breed for cold tolerance, grow out plants in cold conditions. Remove plants that suffer cold damage easily. Breed plants that withstand cold temperatures.
Thin-Layer Chromatography
Thin-layer chromatography can be used to take tests and make selections based upon cannabinoid profiles. Cannabinoid profiles are similar throughout a plant’s life stages, and breeding decisions can be based upon these profiles. For example, the cannabinoid profile can be tested on 2-month-old seedlings. Plants with desirable profiles are kept, and those with undesirable plants are culled.
See “Modern Cannabis Breeding” at the end of this chapter for an overview of marker-assisted selection (MAS).
Influence of Alleles
The phenotypes seen in a given individual are the result of an interaction between the plant’s genotype and the environment. For example, here are 3 phenotypes: short, medium, and tall. Remember, the genotype describes the genetic condition responsible for the phenotype, and to represent it in discussion we assign it symbols.
| Phenotypes | Genotypes |
| short | ss |
| medium | ss |
| tall | SS |
There are always 2 versions of every gene (allele). For example, if there are 2 lower-case “s” plants with “small stature” the plant will be shorter. But if the plant has capital “S” and has the “tall” gene, the phenotype is tall. If both genes are inherited, the plant is medium height.
Homozygous / heterozygous: These are terms used in describing the genotypic condition of a plant, with regard to the similarity of the alleles for a given trait. If a plant is homozygous for a given trait, it has 2 copies of the same allele. If a plant is heterozygous, it has 2 different alleles for a given trait.
The offspring inherits 1 set of alleles from each parent. This inheritance of alleles can be homozygous (both alleles are the same) or heterozygous (each allele is different). Furthermore, recessive alleles do not surface completely for several generations. The influence of alleles makes it impossible to use simple mathematical probability to predict the outcome of offspring.

In August 2011, Dr. Kevin McKernan announced that his company had successfully mapped the genomes (shotgun sequence) for Cannabis sativa (‘Chemdawg’ variety) and later C. indica (‘LA Confidential’). Medicinal Genomics then publicized its work on C. sativa via Amazon’s EC2, a cloud-computing service that gives free access to the scientific community. Search “Cannabis genome EC2 cloud” on www.google.com for more information.

This indica-dominant ‘Peyote Purple’ was developed by CannaBioGen.
Dominant and Recessive Traits
Dominant and recessive traits are dictated by alleles that are inherited from both parents. But, even though the cannabis genome has been decoded, specific gene function has not been deciphered. Consequently, the examples below must be used as guidelines because many traits are driven by a combination of genes.
Dominant: An intra-allelic interaction such that the presence of an allele of 1 parent masks the presence of an allele from another parent plant, in the expression of a given trait in the progeny. Only the dominant trait is shown in the first generation of offspring. Of the F2 generation, 75 percent will also show the dominant condition.
Recessive: An intra-allelic interaction such that an allele of 1 parent is masked by the presence of an allele from the other parent plant, in the expression of a given trait in the progeny. The recessive trait is not shown in the first generation of progeny (F1) but will reappear if siblings are mated, and the F2 progeny will result in 25 percent of plants showing the recessive condition.

‘Afghani’-dominant crosses are in the foreground and sativa-dominant crosses are in the background. (MF)

The ‘Afghani’ seedling on the left demonstrates the dominant traits of short, squat growth and broad leaves. The ‘Kush’ seedling on the right demonstrates the dominant traits of taller growth, and has much different leaf formation.

This genetically purple ‘Mexican’ sativa from 1980 is one of the varieties that added a purple hue to many current varieties. (MF)
Numbers Can Be Misleading
Simplistic math models are often used to explain what happens when male and female cannabis plants are crossed. These models do not take into account dominant and recessive genes, they allow nothing for genotype and phenotype, and they do not consider that specific traits may be controlled by many different genes.
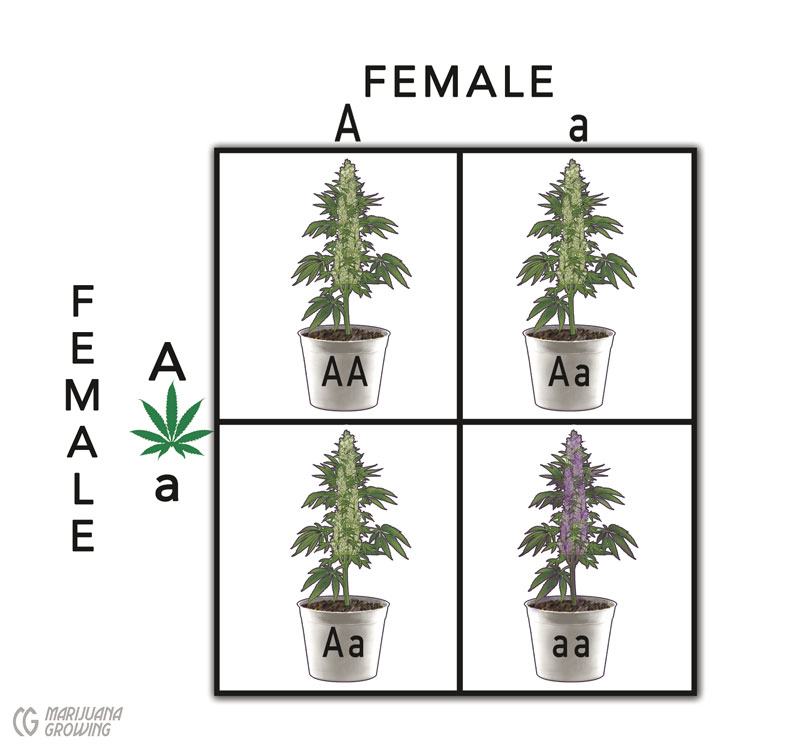
Use the Punnett square to help predict the outcome.
We can demonstrate Mendel’s Laws in a Punnett square to predict the possible outcome of 1 genetic trait (monohybrid). The example below uses Mendel’s pea plant experiment. He crossed 2 yellow pea plants, and they produced 3 quarters yellow peas and 1 quarter green peas. The Punnett square accounts for all the possible combinations for 1 gene. Each of the 4 squares in the box represents 1 new offspring.
A monohybrid is a genetic cross between 2 parents; 1 parent has 2 dominant alleles for a specific gene and the other 2 recessives for the same gene. The offspring, monohybrids, have 1 dominant and 1 recessive allele for that gene. A cross between the offspring produces a 3:1 ratio when grown out of the following (F2) generation of dominant:recessive phenotypes.*
*This example is based on Mendel’s monohybrid pea plant cross. Little information is available to the public about specific gene loci of cannabis. An excellent worksheet is available at BiologyCorner.com (www.biologycorner.com/worksheets/pennygene_key.html).
A dihybrid is a genetic cross between parents that have 2 characteristics controlled by genes at different loci.* The example below shows both parents AaBb and AaBb are heterozygous for both traits (color and height). The possible outcome can be predicted with the help of a Punnett square. The example below is based on Mendel’s dihybrid cross with pea plants. Note the dihybrid Punnett square has 16 different possibilities. This will yield a genotype ratio of 1:2:1:2:4:2:1:2:1 and a phenotype ratio of 9:3:3:1.
The monohybrid cross ratio is demonstrated in the Punnett square.
This example is based on Mendel’s pea plant cross, and is hypothetical because little information is available to the public about specific gene loci of cannabis.

At 4 weeks of flowering, ‘Big Bud’ is just starting to put on weight. The genetics of ‘Big Bud’, from northwestern USA, are found in many of today’s varieties.
True Breed
True-breeding (or inbred) plants make the best breeding stock. A true-breeding variety (aka inbred line [IBL] and true line) of cannabis is the result of a seed lot that has been bred for generations while selecting repeatedly for specific traits. These plants breed true for the specific traits—cannabinoid content, strong growth, desirable aroma and flavor, and so on. There is little or no variation of traits, growth is uniform, and the outcome of future generations is easier to predict. These plants are said to be stable.
If seed is true breeding (IBL) it should be easy to reproduce by open pollination. See “Making Seeds at Home,” page 523, for a list of true-breeding seed varieties. After many generations of repetitive breeding and selecting for the same traits, other genetic traits may deteriorate. See “Inbreeding,” page 518, for more information.
Hybrids
Hybrids are a product of a cross between genetically distinct parents. Hybrids do not reproduce their parent characteristics completely or reliably when reproduced sexually. Develop hybrid cultivars by using inbred (true-breeding) genetics or by segregating populations, inbreeding them, and selecting for hybrid line production. Various hybrid varieties are described below.
Hybrid Crosses
F1 hybrids (‘Northern Lights’ × ‘Blueberry’, ‘Northern Lights’ × ‘Haze’)
3-way crosses (‘Skunk #1’—a cross of (‘Mexican’ × ‘Colombian’) × ‘Afghani’) 4-way or double cross hybrids (cross of 2 unrelated F1 hybrids ‘Haze’ (‘Afghani’ × ‘Thai’) × (‘Mexican’ × ‘Colombian’).
F1 Hybrid Varieties
Achieve an F1 hybrid population by crossing 2 unrelated, true-breeding varieties. F1 hybrids are uniform when grown from seed, but, like all hybrids, they are genetically unstable. If reproduced sexually by inbreeding within the F1 population, the subsequent generation will be neither uniform nor similar to the F1 generation. Seeds from F1 hybrids, F2, will all be different; parents are heterozygous and diverse. They average twice as many monozygous genes and will be less vigorous. Seeds lose uniformity and hybrid vigor.
F1 hybrid vigor (heterosis) occurs when 2 true-breeding varieties are crossed. The resulting seed and plant has “hybrid vigor,”—growing more robustly, stronger, faster, and with a heavier harvest than both parents. For example, a (‘Skunk#1’ × ‘Blueberry’) F1 hybrid grows faster and yields more than either the pure ‘Skunk #1’ or ‘Blueberry’ parent populations.
F1 hybrid seeds grow into strong plants that produce well. Seed companies sell them because they keep clients coming back for more seeds every year. Few seed companies are interested in selling seed that is easy to open pollinate or easy to reproduce. Most seed companies make and release hybrids. Often “true-breeding” (IBL) seeds are not stable. Competitors in the unregulated market continually pirate seed varieties from companies that have developed the seeds.
F1 hybrid is a term used in genetics and selective breeding. F1 stands for Filial 1, the first filial generation of seeds/plants or animals.
Maintaining strong, reliable F1 hybrid stock requires growing 2 different lines of the same parents. The 2 lines are crossed to make seed. Deviation is minimized. This is necessary to maintain genetic diversity and avoid recessive trait domination.
For information on F2, F3, etc., generations, see “Filial Breeding,” on page 520.
Cross-pollination is the transfer of pollen from an individual male cannabis flower to a stigma of the flower of a female plant. Strive to combine the best qualities of a true-breeding line with other lines to increase desired traits when using cross-pollination in cannabis breeding.
Inbreeding
Inbreeding is crossing a group, family, or variety of plants within themselves with no addition of genetic material from an outside or unrelated population. “True Breed,” above, is an example of inbreeding. The most severe form of inbreeding is the self-cross (see “Self-Pollination (Selfing)” on page 520), in which only one individual’s genetic material forms the basis of subsequent generations. And 1:1 hybrid populations are only slightly less narrow, derived from the genetic material of 2 individuals. Such tight or narrow breeding populations lead to a condition called “inbreeding depression” upon repeated self-breeding or inbreeding.
Inbreeding depression is a reduction in vigor (or any other character) due to prolonged inbreeding. This can manifest as a reduction in potency or a decrease in yield or rate of growth. Progress of depression is dependent, in part, on the breeding system of the crop.
Cannabis is an outcrossing or cross-pollinating species. Cross-pollinated cannabis exhibits a higher degree of inbreeding depression when “selfed,” or inbred, than do selfing crops such as tomatoes, which can be selfed for 20 generations with no apparent loss in vigor or yield.
In cross-pollinated crops, deleterious genes remain hidden within populations, and the negative attributes of these recessive traits can be revealed when inbred several generations.
Inbreeding depression can be noticeable in S1 (see “Self-Pollinating (Selfing),” on page 520) populations after a single generation of self-fertilization. When breeding cannabis using small populations, as is often the case with continual 1:1 mating schemes, inbreeding depression typically becomes apparent within three to four generations. This 1:1 breeding model is being used today by most commercial seed banks.
Cannabis is naturally an outcrossing or cross-pollinating species and existed in wild breeding populations of hundreds if not thousands of individuals. Within these many individuals lies a wide range of versions of different genes. When only 1 or 2 plants are selected from this vast array as the breeding population, there is a drastic reduction in the genetic variability found in the original population, resulting in a genetic bottleneck. Once this variability is lost from a population, it is gone.
If space is available, one way to overcome this problem is by maintaining 2 separate parallel breeding lines. After generations of inbreeding, when each of the inbred lines or selfed populations starts showing inbreeding depression, they are hybridized or outcrossed to each other to restore vigor and eliminate inbreeding depression while preserving the genetic stability of the traits under selection.

‘Afghani #1’ is an inbred variety that was developed over years of inbreeding among large populations of cannabis plants. This landrace variety was collected in the mountains of Afghanistan by Sacred Seeds in the 1970s. It was one of the first pure indica true-breeding strains used in worldwide cannabis breeding programs. (MF)
Outbreeding
Outbreeding is the process of crossing or hybridizing plants or groups of plants with others to which there is no relation or the relationship is very distant. Any time a breeder is hybridizing using plants that reside outside of the family, group, or variety, hybrid seed is produced.
An F1 hybrid seed is the first generation offspring that results from crossing 2 distinct true-breeding plants or populations. Each of the (true-breeding) parents was hybridized (outcrossed to each other) to produce the new generation, which is now comprised of genetics from both parental populations. Outcrossing results in the introduction of new and different genetic material to each of the pools.
Often there are recessive genes or dominant genes that have not had a chance to fully express themselves until being inbred 3 to 4 times or being outcrossed several times. It is important to remember that cannabis is naturally outcrossing. Inbreeding plants that are naturally outcrossing too many generations sacrifice the health of offspring. Keep outcrossing plants healthy, and maintain diversity by growing large numbers of plants.
Filial Breeding: Siblings of the same progeny lot and generation are interbred to produce new generations. The first hybrid generation of 2 distinct true-breeding lines is the F1 generation (F, filial). If 2 F1 siblings are bred, or the F1 population is allowed to be open-pollinated, the resulting generation is labeled F2, F3, F4, etc. Mating siblings chosen from the F2 results in the F3 population. F4, F5, F6 generations, etc., are produced the same way, by crossing plants of the same generation and progeny lot.
Note: As long as any number of siblings of a generation (F[n]) are mated, the resulting generation is denoted (F[n+1]).
Filial inbreeding with selection for specific traits is the most common method for establishing a pure or a true-breeding population in cannabis. After a few generations, genetic problems will surface.

‘Super Skunk’ from Sensi Seeds is a good example of an outbred variety. It takes the stable ‘Skunk #1’ and adds a dominant amount of ‘Afghani’ genetics.
Backcrossing
Backcrossing is the pollination of a female flower from a male flower on the same plant. Backcross breeding is repeated crossing of progeny with one of the original parental genotypes. It is cross-pollinating 1 generation back to a previous generation; most often the progeny is crossed with the mother plant. The parent is called the “recurrent parent.” The nonrecurrent parent is the “donor parent.”
Backcross breeding is the most widely used form of breeding cannabis to date. Backcross breeding is simple and can be done with small populations of plants. Most often, the goal of backcrossing is to create a population from the genetics of a single parent (the recurrent parent).
Donor parents are chosen based on desirable traits. One parent is backcrossed to another to introgress genetics, to move genes from one variety to another. Repeated backcrossing is the best way to achieve this goal.
Use backcrossing to add desirable traits to a mostly ideal, relatively true-breeding genotype. The recurrent parent should be an ideal genotype such as an existing inbred line. Look for traits that are easily identified in the progeny of each generation. The best donor parent must possess the desired trait but should not be seriously deficient in other traits. Backcross line production is repeatable if the same parents are used.
Simple backcrossing to incorporate a dominant trait is easy and very common among the vast majority of breeders. However, plants come with both dominant and recessive genes. When making selections, it is best to select for one single quality at a time and select the absolute best males possible from each population.
Backcrossing also uses the terms “squaring” (to denote the second backcross to the same parent) and “cubing” (to designate the third backcross).

‘Apollo 13 BX’ (Back Cross) from TGA Genetics is a good example of a backcrossed variety.
Backcrossing: Incorporating a Dominant Trait
Step One: Cross recurrent parent × donor parent
Step Two: Select desirable plants showing the dominant trait, and hybridize selected plants to the recurrent parent. The generation produced is denoted BC1 (some cannabis breeders call this generation B×1 [BC1= B×1]).
Step Three: Select plants from BC1 and hybridize with the recurrent parent; the resulting generation is denoted BC2.
Step Four: Select plants from BC2 and hybridize with the recurrent parent; the resulting generation is denoted BC3.
Backcrossing: Incorporating a Recessive Trait
Recessive traits are more difficult to select for in backcross breeding since their expression is masked by dominance in each backcross to the recurrent parent. An additional round of open pollination or sib-mating is needed after each backcross generation to expose homozygous-recessive plants. Individuals showing the recessive condition are selected from F2 segregating generations and backcrossed to the recurrent parent as in the “Backcrossing: Incorporating a Dominant Trait” above.
Step One: Cross recurrent parent × donor F1 hybrid generation.
Step Two: Select desirable plants and create an F2 population via full cross-pollination.
Step Three: Select plants showing the desired recessive trait in the F2 generation. Hybridize selected F2-recessive plants to the recurrent parent. The generation produced is denoted BC1.
Step Four: Select plants from BC1 and create a generation of F2 plants via sib-mating; the resulting generation can be denoted BC1F2.
Step Five: Select desirable BC1F2 plants showing the recessive condition, and hybridize with the recurrent parent; the resulting generation is denoted BC2.
Step Six: Select plants from BC2, and create an F2 population via sib-mating; denote the resulting generation BC2F2.
Step Seven: Select plants showing the recessive condition from the BC2F2 generation, and hybridize to the recurrent parent; the resulting generation is denoted BC3.
Step Eight: Grow out BC3, select and sib-mate the most ideal candidates to create an F2 population, where plants showing the recessive condition are then selected and used as a basis for a new inbred, or open-pollinated, seed line.
This new generation created from the F2 is a population that consists of, on average, about 93.7 percent of genes from the recurrent parent, and only about 6.3 percent of genes leftover from the donor parent.
Note: Only homozygous-recessives were chosen for mating in the BC3F2 generation; the entire resulting BC3F3 generation is homozygous for the recessive trait and breeds true for this recessive trait. This new population meets the breeding objective, consisting mainly of genetics from the recurrent parent while breeding true for the introgressed recessive trait.
Backcross-derived lines are most often well-adapted to the environment in which they will be grown. Indoor garden rooms are easily replicated, and plants grow in a similar environment to that in which they were bred. Progeny therefore need less extensive environmental field-testing.
If 2 or more characters are to be introgressed into a new seed line, these would usually be tracked in separate backcross programs, and the individual products would be combined in a final set of crosses after the new populations have been created by backcrossing.
Backcrossing has some drawbacks to consider. If the recurrent parent is not a very true-breeding variety, the resulting backcross generations may segregate, and many of the desirable traits intended to be reproduced in the line may fail to be replicated reliably. Additionally, the limitation of backcrossing is that the “improved” variety differs only slightly from the recurrent parent, typically focusing on one specific trait. If the goal is to introgress multiple traits into a new population, other breeding techniques like inbreeding or recurrent selection might be more effective and rewarding.

Small male flower appears on this Chemdog inbred female flower. Inbreeding often causes male intersex flowers to form on female plants.
Self-Pollinating (Selfing)
“Clone Only” Varieties Often, 2 hybrid plants are crossed and the “variety” is given a name, but soon the male plant is lost, and the plant is available only as a clone. In this case the plant must be “selfed” to produce male flowers on a female plant.”
Self-pollinating (aka selfing) is the process of creating seed by pollinating a plant with its own pollen. Self-pollinating is a plant having sex with itself. Self-crossing can derive populations of plants from a single individual. The first generation population derived from selfing an individual is called the S1 population. An individual from S1 that is selfed again is called S2. Subsequent generations derived in the same manner are denoted S3, S4, etc.
Traits for which the plant is homozygous remain homozygous upon selfing, whereas heterozygous loci segregate and may demonstrate novel expressions of these characters.
We know homozygous loci remain homozygous in future generations upon selfing. Heterozygous loci are increased by 50 percent. Every subsequent generation will be 50 percent more homozygous than the parent from which it was derived.
Repeated selfing, or single-seed descent, is the fastest way to achieve homozygosity within a group or family. The more plants grown from a selfed population, the better probability a breeder has of finding selfed progeny that show all the desired traits.
Self-Pollinating Breeding
Step One: Identify superior genotypes for the trait under selection.
Step Two: Cross superior genotypes and select improved progeny.
Step Three: Repeat steps One and Two over a series of generations.
Feminized Seeds
Breeders produce all-female or feminized seeds by obtaining pollen from a male flower on an individual female and using this pollen to fertilize another female plant. In order to produce feminized seed, pollen must be collected from male flowers that occur on an otherwise female plant.
As stated in “Sexual Propagation,” there are 20 chromosomes in each plant cell. Female cannabis plants have 2 copies of the X chromosome, and their genotype is expressed as XX. Male plants have 1 copy of the X chromosome and 1 copy of the Y chromosome. The genotype expression of male plants’ sex chromosomes is XY. However, the ability to change sex based on external factors is believed to be controlled by X-autosome or X-A.
When pollen is created within the plant, 1 of each of the sets of chromosomes from each parent is packaged into the cells that develop into pollen. This process, mitosis, is the process of a single cell dividing into 2 identical cells. Each cell contains the same chromosomes and genetic content as the parent. Each pollen grain or ovule contains 10 chromosomes, 1 copy of each pair. When the pollen deposits the genetic material into the ovule, the 10 chromosomes from the pollen and the ovule unite to make a total of 20 chromosomes, a full genetic complement. There are 2 gametes: 1 fertilizes the ovule of the female, the other fertilizes the endosperm of the seed that gives the new plant its initial food source and chemical profile.
Use a Punnett Square to predict the outcome of a breeding cross. It is used to determine the probability of an off- spring having a particular genotype. The Punnett square summarizes all possible combinations of 1 maternal allele with 1 paternal allele.
The next step is to produce male flowers on a female plant. Pollen from the male flowers is used to fertilize the same female plant (selfing) or other female plants (outbreeding). If this female plant is the same self-pollinated plant, it will be a mix of its own genes. If the plant is different and from outside the genetic pool of the first plant, new genes will be introduced to the outbred cross.
There are 2 common ways to induce male flowers on a female plant— inducing environmental stress and altering hormone levels.

Seeds must develop completely before they are ready to harvest. (MF)

‘Sour Bubbly’ is one of the many new femi- nized autoflowering varieties.
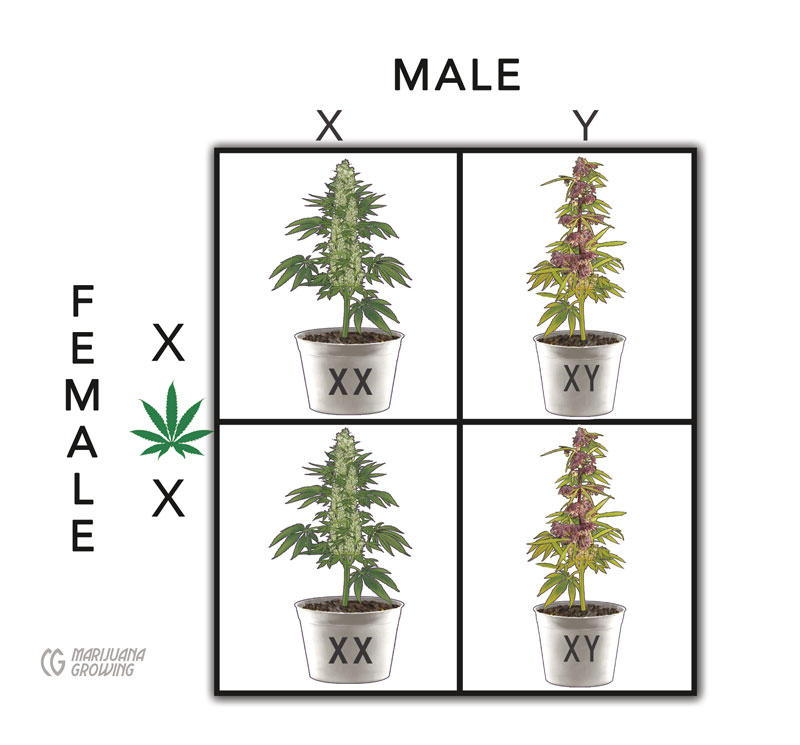
Punnett square view of a simple male:female cross
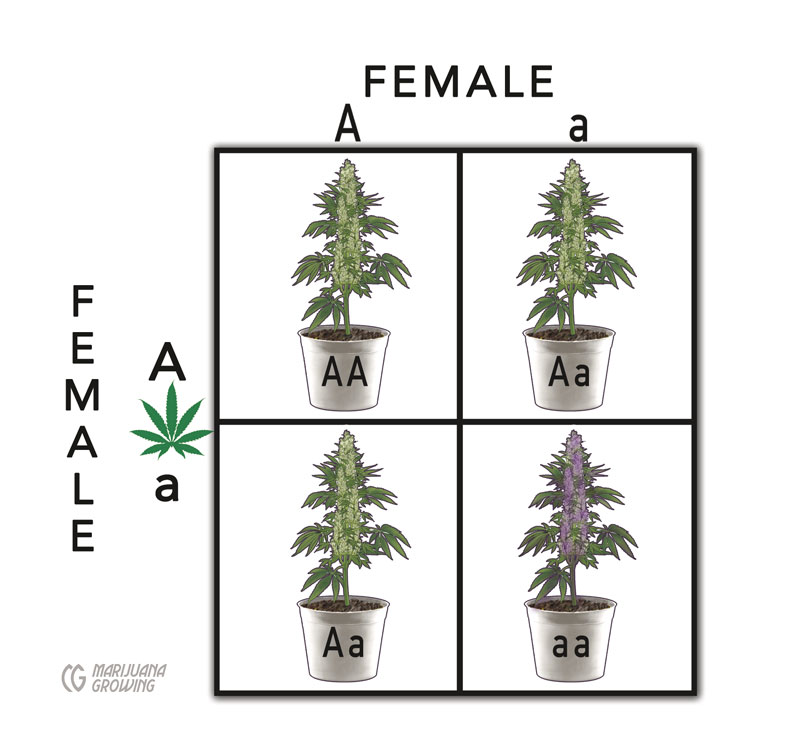
This Punnett square of a female cross demonstrates that female:female breeding crosses produce only female (XX) offspring. Pollen from a few male flowers that appear on a female plant is used to produce female only (XX) offspring.
Environmental Stress
Pollen from environmentally stressed intersex plants is used to fertilize females that will produce mostly (more than 99%) female seeds. But, the offspring will have intersex tendencies. More pronounced intersex tendencies in parent plants bring about the same traits in offspring. There is no way around this simple genetic fact.
Produce feminized seed by collecting pollen from carefully selected, latent, stress-induced male flowers on a female plant, and use it to pollinate female plants.
One of the easiest stress techniques is to let the plant flower and continue growing. Sooner or later most females will produce a few male flowers. Other stress methods include irregular light cycle or low temperature. The process is time-consuming but yields mostly female plants when grown without stress. As soon as stress is introduced, intersex tendencies can quickly surface.
Remember, genetic weaknesses— including a predisposition for male flowers to appear on female plants—will be passed to offspring. To select against the intersex condition, take female breeding candidates and grow them under stressful conditions such as irregular light cycle or high heat. Only plants that resist intersexuality under stressful conditions should be tested to produce an all-female seed lines. Intersex-resistant plants are called “true females.” Always select females that resist changing sex to pass the trait to offspring.
Environmental stress is not the only way to produce male flowers on a female plant.
Alter Hormone Levels
Feminized seeds can also be produced with applications of sprays that alter the hormonal concentration of the plant. There are 2 popular methods of inducing male flowers on a female plant. Both methods require a spray application to the plant.
Colloidal silver (CS) consists of very small silver particles suspended in water. There is quite a bit of disagreement as to the overall safety and efficacy of colloidal silver. Please research safety aspects before using it. In cannabis, CS inhibits female flowering hormones, so male hormones dominate. Male flowers appear on an otherwise female plant. Purchase high-quality CS at health food stores, natural pharmacies, or via Internet stores. Look for CS that is 15 to 30 ppm concentration. Or you can make it yourself at home, which is a bit more complicated. There are many YouTube videos and Internet methods to follow. They involve using a 9- to 12-volt battery or generator that can produce at least 250 milliamperes, a silver rod or coin, and distilled water. For instructions on colloidal silver, see chapter 22, Additives.
Silver thiosulfate is more difficult to find than CS, but it is available on the Internet. It works on the same principles as CS. Check package for spray concentration and application.
Gibberellic acid (GA3) is a very popular hormone used to produce feminized seeds. See chapter 22, Additives, for complete information.
Ethylene, a plant hormone, plays a major role in the determination of sex by regulating which flowers should be produced–male or female. High concentrations of ethylene sprayed on male flowering plants causes female flowers to form. See chapter 22, Additives, for more information on ethylene.
Applying ethylene-inhibiting agents to pistillate individuals as they enter flowering results in the formation of stamens in place of pistils. Breeders use this technique to create “feminized” seeds (all-female [gynoecious] seed lots).
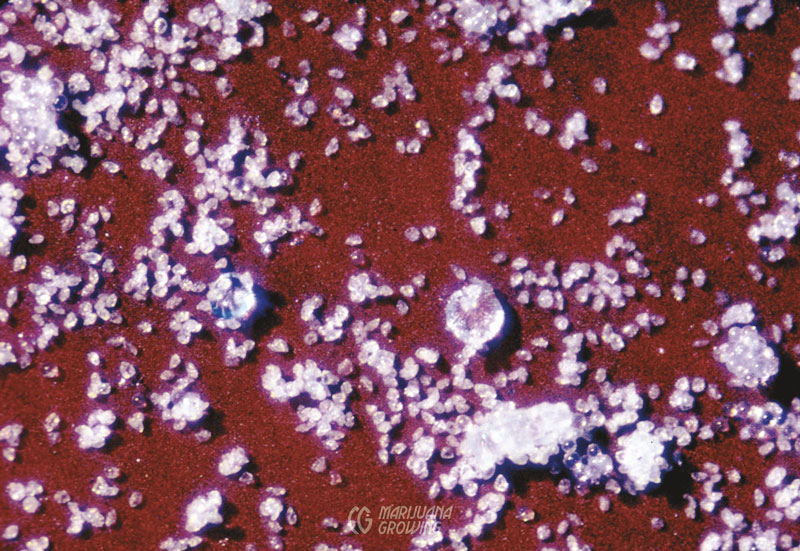
Large, round, capitate-stalked resin gland heads add perspective to minute grains of male pollen. (MF)

This autoflowering feminized ‘Big Low’ from Seeds of Life is one example of the many new “auto-fems” available today.

‘Jack 47’ from Sweet Seeds proves that autoflowering feminized seeds produce plenty of resin.
Day-Neutral (aka Autoflowering) Feminized Varieties
Day-neutral cannabis plants are called autoflowering plants in the popular cannabis industry today. Autoflowering feminized seeds are produced the same way as other seeds, except 1 or both of the parents are autoflowering. These varieties were originally created by cross-pollinating Cannabis ruderalis that flowers after 3 to 4 weeks of growth with regular cannabis plants that flower under 11 to 12 hours of darkness after the vegetative growth stage is complete. Autoflowering varieties are relatively easy to create, but they take time to stabilize. Seed companies sell them to you every year so that you do not have to reproduce them.
Breeding new autoflowering varieties is more difficult when making hybrids and incorporating a non-autoflowering variety. A few short-day cannabis varieties are heterogeneous, contain the recessive day-neutral (autoflowering) genes and dominant short-day genes.
Autoflowering plants have homozygous recessive traits for the day-neutral genes. Most short-day varieties and autoflowering crosses produce few autoflowering progeny in the F1 generation. When recrossed, the F2 generation contains approximately 25 percent of homozygous recessive plants that are autoflowering. Once this point is reached, the population requires further stabilization.
Learn to breed with regular seeds before starting to breed autoflowering feminized plants. Once you have a good background with the variety you are breeding, you can start autoflowering breeding. Remember, half the equation is finding a male that fits your needs and is super strong-smelling, resinous from an early age, disease- and pest-resistant, and so on. Such males are often short and squat, with female-like growth and stature characteristics.
Once autoflowering plants are genetically stable, they are feminized via inbreeding pollen from a male flower that occurs on a predominantly female plant. The process is fairly simple and is outlined below.
Breeding Basics for Autoflowering Feminized Plants
- Cross regular seed with an autoflowering variety.
- Cross F1 seeds together again under a 12/12 photoperiod.
- The first generation contains recessive autoflowering genes that manifest in the next generation.
- The next generation has some autoflowering genes.
- Plant and stabilize over time to get 100 percent autoflowering plants.
- Feminize 100 percent autoflowering female and produce seeds.
Backcross regular seeds to an autoflowering male to achieve an autoflowering plant too.
Breeding new autoflowering varieties is more difficult when making hybrids and incorporating a non-autoflowering variety. A few short-day cannabis varieties are heterogeneous, contain the recessive day-neutral (autoflowering) genes and dominant short-day genes.
Autoflowering plants have homozygous recessive traits for the day-neutral genes. Most short-day varieties and autoflowering crosses produce few autoflowering progeny in the F1 generation. When recrossed, the F2 generation contains approximately 25 percent of homozygous recessive plants that are autoflowering. Once this point is reached, the population requires further stabilization.
Learn to breed with regular seeds before starting to breed autoflowering feminized plants. Once you have a good background with the variety you are breeding, you can start autoflowering breeding. Remember, half the equation is finding a male that fits your needs and is super strong-smelling, resinous from an early age, disease- and pest-resistant, and so on. Such males are often short and squat, with female-like growth and stature characteristics.
Once autoflowering plants are genetically stable, they are feminized via inbreeding pollen from a male flower that occurs on a predominantly female plant. The process is fairly simple and is outlined below.
Breeding Basics for Autoflowering Feminized Plants
- Cross regular seed with an autoflowering variety.
- Cross F1 seeds together again under a 12/12 photoperiod.
- The first generation contains recessive autoflowering genes that manifest in the next generation.
- The next generation has some autoflowering genes.
- Plant and stabilize over time to get 100 percent autoflowering plants.
- Feminize 100 percent autoflowering female and produce seeds.
Backcross regular seeds to an autoflowering male to achieve an autoflowering plant too.
Making seeds at home requires a secure garden area that can be dedicated to breeding. It must be clean and free of male pollen. Indoor garden rooms and greenhouses are best suited to breeding cannabis if there is danger of rogue pollen from neighboring male cannabis plants contaminating faraway seed crops. If growing more than 1 male to produce pollen, measures must be taken to isolate each male once they start producing pollen. Segregate pollen-producing male plants by keeping them in an enclosed area as far away from flowering females as possible.
The basics of breeding cannabis at home are simple:
- Start with a diverse group of plants and know their traits.
- Cross desirable plants.
- Select desirable individuals and maintain diversity.
Slow and steady breeding is not dynamic, but it is very effective. Pollinate a few branches on several plants and have more seeds than you can grow. The breeding project is never finished!
Grow small seed crops inside a garden closet or inside a portable wardrobe made from fine cloth or something similar that will block pollen from entering or escaping.
Recordkeeping is the most important aspect of cannabis plant breeding. Accurate written and photographic records with dates help you to make informed decisions.
Naming Varieties
Many varieties have names known only in the local area where they are cultivated. These varieties often bear the name of the region from which they originated or a person with whom they are associated. Other varieties are named for fragrance, bud size, effect, or appearance. The names are often very descriptive and creative!
Making Seeds: Step-by-Step
Step One: What is your goal? Common goals include making seeds for next year’s crop, reproducing new parents just like the old ones, and adding new traits to existing plants. When setting a goal, work with a single trait at a time so that it is easier to control the selection and outcome. Breeding will bring about dominant and recessive genes. Each has its own qualities. Impeccable recordkeeping and a consistent, stable climate are essential. Write out a breeding plan and plot your findings.

‘Skunk #1’ is a stable breeding plant and is at the base of many varieties and breeding programs. Choose stable breeding stock whenever possible. (MF)
Step Two: Select parents—(A) female, (B) male—to breed. Few varieties commercially available to medical cannabis gardeners are true-breeding and stable. Most often seeds have many different genes and are not stable or uniform. True-breeding seed stock ensures F1 hybrid seeds that have hybrid vigor. Stable true-breeding stock is sometimes difficult to find from seed companies, and gardeners often elect to stabilize their own.
Simply crossing 1 of the stable true-breeding varieties (see list below) with another stable variety will produce an F1 hybrid.
List of relatively stable seeds:
- ‘Afghan#1’ (IBL)
- ‘Big Bud’ (IBL)
- ‘Blackberry’ (VISC)
- ‘Burmese’ (VISC)
- ‘Durban Poison’ (IBL)
- ‘Hash Plant’
- ‘Hindu Kush’
- ‘Island Sweet Skunk’
- ‘KGB’ (VISC)
- ‘Malawi Gold’ (IBL)
- ‘Master Kush’ = stabilized hybrid
- ‘Original Blueberry’ = stabilized hybrid
- ‘Original Haze’ (IBL)
- ‘Power Plant’ (IBL)
- ‘Skunk Passion’ (IBL)
- ‘Skunk #1’
More stabilized seed stock is updated at www.marijuanagrowing.com

This beautiful medical ‘Chronic’ female is an excellent candidate for a breeding program.
Step Two A: Select a female that has been flowering for about 4 weeks and has many white stigmas.
Female plants are easy to select. Medical cannabis gardeners and patients know the growth characteristics of each plant in the garden. The most common desirable characteristics include cannabinoid profile with quality fragrance and flavor, strong growth habit, heavy yield, and resistance to diseases and pests. Outdoor gardeners in climate-sensitive areas look for strong branching, a big root system, and tolerance of drought, heat, and cold.
Take a thin-layer chromatography test to help make correct choices based upon cannabinoid profiles. Cannabinoid profiles are similar throughout different life stages of a plant, and breeding decisions can be based upon these profiles.
Observe plants carefully as they develop during the season. Watch for desirable characteristics. Pay close attention to flower buds and how they fill out and mature. Apart from looking at resin gland development and stigma senescence, take a toke. Vaporize a sample bud from each plant to determine taste, fragrance, effect, and so on. Remember that flower bud flavors and aromas can change over time as they dry and cure. Additional tasting may be necessary.

‘Hash Plant’ × ‘G13’ × ‘Chronic’ yielded this pistil-heavy female. Although showy, the breeder culled the plant in favor of others with heavier bud development.
Step Two B: Select a male with desirable characteristics.
Choosing male plants for breeding is difficult. One basic test is to rub the stem with your finger. If it exudes a pungent, resinous odor, it may be rich in cannabinoids. Look for male plants that are short with dense foliage and that contain other desirable characteristics—cannabinoid profile, strong growth, disease and pest resistance, drought, heat, and cold tolerance.
To determine cannabinoid profiles, make thin-layer chromatography tests before males flower. Soon laboratory analysis of the cannabinoid profile and genetic marker (alleles) tests will become more common.
The most reliable way to select the best male breeding stock is to use the pollen from specific males to pollinate chosen females. The plants are grown to maturity, and males are then selected. It takes time to stabilize males, and few seed companies put much time into male plant selection. Once selected, a super male and subsequent male lineage can be kept alive for years.
Clone promising male plants and cull the rest to keep stock alive.

Testing male plants with thin-layer chromatography is a good way to learn much about the cannabinoid profile. Without the test, cannabinoid profile would have to be measured subjectively.

Waiting for a male plant to mature is difficult when growing females in the same vicinity.
Step Three: Collect pollen.
Set up small “plates” of aluminum foil to collect pollen. Once plates are in place, jiggle branches so pollen is dispersed and falls onto plates for collection.
Carefully collect pollen from male flowers and store it in an airtight container in a cool dry place or in a low-humidity refrigerator or freezer. Or cut a male branch from a plant and store it in a glass of water in subdued light.
Pollen can be stored for several years if kept cool and dry. Viability decreases as time passes. But consider that there are millions of grains of pollen in a single male flower. It takes just a fraction of the pollen to fertilize an entire bud. Even though pollen has lost viability, there is still enough good pollen to fertilize pistils and produce seeds. One branch of male flowers will supply all the pollen necessary for small-scale breeders to produce many seeds for personal use.
To avoid premature pollination, isolate the male as soon as anthers show. Be considerate of the fact that airborne pollen can travel miles. Brushing up against a plant jostles and releases millions of grains of pollen that travel everywhere.

Pollen is being collected on lightweight aluminum foil. (MF)
Step Four: Store pollen.
Store small amounts of pollen in aluminum foil, enclosed with silicon to absorb excess moisture so that it does not destroy the pollen. Pollen has a short shelf life in nature. High temperatures and moisture destroy it. Pollen can be stored in the freezer for several months.
Store pollen in an airtight container along with silicon pellets to absorb any moisture in the container. Periodically remove silicon to dry and then be returned to the container. Make sure to let the container sit at room temperature for several hours to avoid moisture condensation from temperature change.

Carefully remove pollen from the collection bag and pass it through a screen to remove flower remnants. Remember, dead flowers and foliage attract moisture and will contaminate and spoil pollen. Place wax paper under the screen to catch pollen. Pour pollen into a sterile test tube or small container from a craft store. Scrape remaining pollen into the tube or container with a sterile instrument. Seal the test container. Put the test tube in a larger airtight container with several packets of silica gel to absorb moisture. Seal pollen with silica gel and leave at room temperature so the silica draws moisture from pollen before putting it in the freezer.

At pollination time, pull pollen out of the freezer and let it warm up to room temperature. Do not open the container when cold or water will condense inside and kill the pollen. Keep the refrigerator at low humidity. Thawing and refreezing pollen will diminish its viability.
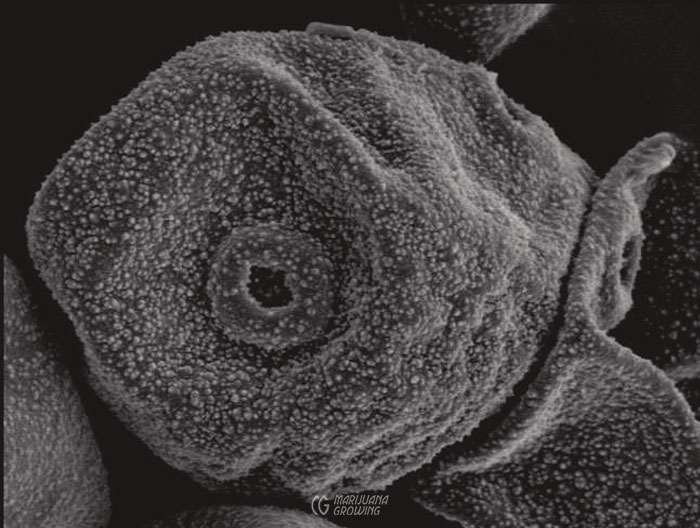
Grains of male pollen such as the one in the photo (magnified 4,000 times) stick to the stigma, develop a tube through the style, and release 2 gametes—1 to fertilize the ovule and 1 to fertilize the endosperm.
Step Five: Pollinate female.
Flowering females grow many ready, receptive stigmas until pollination occurs. Best pollination takes place about 3 to 5 weeks after females show their first flowers. At this point the majority of receptive stigmas are ready for pollination. This is when a few of the stigmas start to curl and slightly discolor, signifying the onset of senescence. Receptive stigmas are turgid and most often are white or off-white in color.
Senescing and dying stigmas that are brownish are not viable.
Not all pollinations are successful. If in doubt, you may want to pollinate 2 or 3 times to ensure your success.
Once pollinated, the majority of the female’s energy is directed toward seed production, and bud formation stops. Pollinate well-formed buds packed with well-formed stigmas to produce the maximum number of viable seeds.
Home breeders need to pollinate a flower bud full of stigmas or a single branch full of buds to make more seeds than they need. Humboldtlocal calls pollinating one branch “planned parenthood.” The unpollinated branches are sinsemilla. The sinsemilla tops are harvested when ripe. Seeded branches continue to mature for another week or two until ripe.
A little bit of pollen on your thumb or finger is the best way to pollinate specific sets of stigmas in a flower bud. Human skin is oily and it holds pollen well without altering it. Brush pollen-laden thumb or finger lightly to disseminate countless grains of pollen to fertilize females. Be very careful with remaining pollen on the finger. Rinse fingers in water to remove pollen, or quickly lick pollen with your tongue.
Label everything. It is easy to lose track of names. Cross-reference notes or codes on plants with copious notes in a notebook. Color-code pollinated females with small pieces of yarn for easy identification. Some breeders use barcodes to track plants. The barcode is fixed onto the plant tag and attached to the plant.
Put a little pollen into a plastic bag. Carefully cover a single female branch packed with receptive ripe stigmas with the bag. Tie the end of the bag around the stem of the branch to ensure that no pollen escapes. Shake the branch lightly for complete dispersal of pollen to all stigmas. Leave the bag for 2 days and nights to ensure thorough pollination.
Move the target plants to a “safe” area to avoid accidental pollination of other plants. Be careful not to scatter pollen when removing the bag.
Avoid using an artist’s paintbrush to pollinate individual buds. Pollen is very fine; a paintbrush tends to hold too much pollen, and it is difficult to contain. Pollen can easily disperse beyond the targeted stigmas.
Some commercial breeders and seed makers place multiple males or clones of the same male in the seed production garden room with their breeding females. Males release pollen in the well-vented room, allowing complete pollination of the crop.
Wash clothes and shower afterward to avoid transporting pollen to nearby plants away from the breeding zone.
Spray pollinated females with water a couple of times before returning them to the normal “sinsemilla” grow-room population.
Clean seed room thoroughly after each seed crop to avoid accidental pollination of future crops.

Male flowers can be stored for a few days in a glass of water, but they have a tendency to open prematurely.

Cannabis is very promiscuous! Michael from www.SickMeds.com demonstrates a special bag with a window to prevent pollination. Paper bags are good indoors and in greenhouses; waterproof plastic may be necessary outdoors.

A single lower branch of this big female is fertilized with male pollen. The seeds are left to mature for about 2 weeks after flower buds have been harvested.

A little dab of pollen is all that is necessary to selectively pollinate a few stigmas.
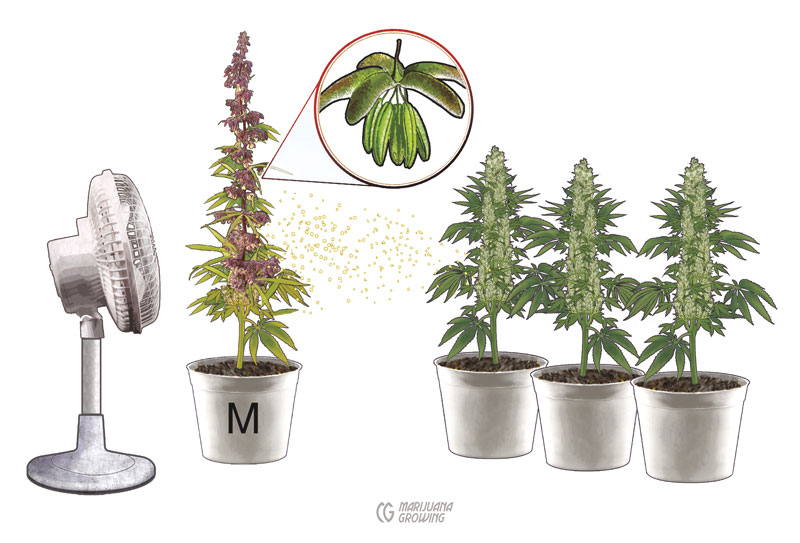
Set a group of females in an enclosed area. Set a single male plant a few feet from an oscillating circulation fan. Turn on the fan to help disperse pollen in the room to pollinate all the females.
.
Step Six: Harvest seeds.
Seeds are ripe when they are mostly dark brown or gray. Many seeds are spotted and mottled; some are even tiger-striped. Green, yellow, or white seeds are almost always immature and not viable. Seeds that are lighter in color or float in water after 24 hours are slow to germinate and often unviable.
When a few seeded buds are mature, remove seeds from the bracts by picking seeds out by hand. This can be done with green buds or dry seeded flower buds. Once dry, rub seeded buds between your fingers and hands over a screen or tray. A little bit of light friction will not damage seeds. However, they are susceptible to heat, cold, and moisture. Avoid all three when harvesting and handling seeds.
Note: Do not use excessive heat or a microwave to dry seeded buds.
Collect seeds and foliage on the tray or screen. Move the tray back and forth so that lightweight foliage stays on one side as the tray is tipped and seeds congregate on the other end. Remove excess foliage by hand and repeat the process. Rub separated seeds together gently in your hands to remove traces of seed bracts that still adhere to seeds. If not collecting resin, use canned air, a fan, or your breath to blow away small amounts of lightweight foliage.
Test for ripeness by pressing a few seeds between your thumb and finger. Seeds that do not crush easily are most often viable.
Under humid and rainy conditions, ripe seeds can actually sprout in place, in flower buds. Once moisture enters the outer shell of the seed, it starts to germinate. Or if foliage dries around seeds and they fall to the ground, they often germinate on the soil surface. But most often they are planted outdoors and consumed by birds or many other hungry creatures.
Seeded flower buds can contain as much resin as sinsemilla flower buds. However, much resin is degraded during the harvest process. Resin glands are broken or resin sticks to fingers and is exposed to excessive light and air when seeds are removed. Seeds are often cleaned over a screen so that the resin powder can be collected below.

Once pollinated, female plants produce seeds that become ripe in 6 to 8 weeks. (Occasionally they mature later.) As seeds mature, they become darker. Eventually, the containing seed bract starts to senesce and dry out around the edges to expose the maturing seed within.

Cleaning seeds by hand can be a laborious task. (MF)

This seeded bud of ‘Afghani’ × ‘Chiba Colombian 60’ × ‘African 3’ was grown as a seed crop in 1980. (MF)

The same bud from above held 50 seeds. (MF)

Seed-cleaning devices save hours of work. The seeded buds are placed on top of the upper screen. Human seed cleaners rub buds together between their hands. The friction causes seeds to separate from seed bracts and buds. Cleaned seeds are collected below in a pan.

Seeded ‘Diesel’ female (MF)
Step Seven: Store or plant harvested seed.
Seeds are ready to plant immediately after harvest and will germinate well. Maintain high germination rates by letting seeds dry in a cool, dark, well-ventilated place after harvest. Seeds that dry for a month or longer before planting develop a hard outer casing to protect the embryo inside. See “Storing Seeds” in chapter 5, Seeds & Seedlings.
Place harvested seeds in a glass or rigid plastic container with a desiccant. Label and date the container. Store seeds in a cool, dry, dark place. Change the desiccant every month or two.

Plenty of silicon desiccant and small containers are needed to store a variety of seeds.
Step Eight: Grow and evaluate.
Growing more plants means greater variation and a bigger pool of individuals. Undesirable plants are easy to cull in order to provide more space for adjacent plants to grow.
Felix from Owls Production, Switzerland, culled all plants that did not meet his requirements for tolerance to cold and mold. This simple technique worked very well. Only the strongest individuals survived the selection process.

A Canadian friend near Montreal sent me this photo. He was fantasizing about making breeding selections. Unfortunately, this is a field of industrial hemp!
Criteria:
- Cannabinoid profile
- Growth habit
- Susceptibility to diseases and pests
- Climatic conditions for acclimatization
- Genetic stability
Step Nine: Monitor, select, and apply selection pressures.
Post-harvest selection requires either partial seeding of each plant or keeping clone copies of each and every plant for future seed-production use once post-harvest evaluations are done.
Pursue Goals:
- Desirable characteristics—cannabinoid profile
- Overall vigor and good health—growth habit, susceptibility to diseases and pests
- Genetic stability
- Acclimatization to your climate and conditions
Varieties for indoor cultivation: These include short, squat, bushy growth; large, densely formed buds; discernible taste or particular flavors and aromas; high content of specific cannabinoids, and quality of the effects (long-lasting, soaring, sedative); and resistance to diseases and pests.
Often, varieties that grow well indoors will also perform well in a greenhouse and outdoors after 2 to 3 years of acclimatization. During the first year in which varieties are moved from an indoor environment to an outdoor garden or greenhouse, some will grow better than others and acclimate more quickly. However, varieties that grow well outdoors often grow poorly indoors, especially pure sativa and sativa-dominant varieties.
Greenhouse varieties grow well in containers. Both outdoor and indoor varieties often grow well in greenhouses. Look for robust growth habit and early flowering if plants are grown in containers. Plants grown in Mother Earth in the floor of the greenhouse must mature before they consume 75 percent of the space inside the greenhouse. Air circulation and ventilation, as well as diseases and pests, become problematic if plants grown in greenhouses become too big.
It is especially important for outdoor varieties to be acclimated to local conditions. Heat, humidity, rain, and cold temperatures are the main aspects of concern among outdoor breeders. Outdoor varieties should be acclimated to the local climate before selecting plants. Smart breeders select plants that mature early in their climate. Pragmatic breeders select the earliest plants with a desirable cannabinoid profile. Selecting for an early harvest without considering cannabinoid content is counterproductive.
It is important to balance positive and negative aspects of each plant and future generations. Selecting plants that may have some genetic weaknesses will require that the unwanted negative traits are removed later.
When planting a large number of seeds, some traits of plants may differ greatly, but in all other respects, they are virtually identical. For example, some plants may be more or less susceptible to fungal infections such as Botrytis (gray mold) or powdery mildew.
Plants can be exposed to a particular pathogen or environmental stress as a selection pressure. Growing potential breeding parents in a stressful environment may expose genetic strengths and weaknesses associated with a particular environment. Heat- and cold-tolerant plants may experience excessive predator attacks or be weak and susceptible to pathogens. Very little selective breeding has been done to preserve cannabinoid profiles and tolerance for diseases and pests.

“This unknown outdoor variety was developed in Northern California to grow well in a localized climate.”

Hasta la fecha, se ha realizado muy poco cruce para desarrollar variedades de cannabis resistentes a enfermedades y plagas. El mildiú polvoriento sigue siendo uno de los principales problemas entre los cultivadores de cannabis.

“Granddaddy Purple” es una variedad de cannabis ampliamente conocida.
Seed-Crop Care
Cannabis grown for seed production requires a complete balanced diet from seed germination through harvest. Give seeded mother plants a complete fertilizer that supplies all nutrients. Often vegetative and flowering fertilizers are mixed together and applied from an early age. I prefer to use complete, balanced, organic-based soil mixes to produce the most healthy, viable seeds.

Growing seed crops in a greenhouse is a good way to keep them isolated from other plants.
Breeding Terms
A grasp of basic breeding terms is necessary to full understanding of essential concepts in this chapter. The breeding terms that follow are easy to look over quickly and decide which ones to study in detail.
A variety*—is a subdivision of a (cannabis) species comprised of naturally occurring or selectively bred populations or individuals that have distinct characteristics from all other varieties in the species. The variety must be uniform and stable under normal growing conditions. However, minor variations may be apparent.
A cultivar (cv)* is a distinguishable plant or group of like plants selected for desirable characteristics, and maintains distinguishing traits when propagated. Most cultivars are selectively bred but some are selections from the wild. The terms cultivar and variety are equivalent.
Unique plants selected from a group or population derived from a cultivar that show enough variation from the parent are considered a different variety and deserve their own name.
*According to some taxonomists, using the term strain to define variety or cultivar is botanically incorrect. For more information, see chapter 8, Flowering.
Open-pollinated varieties are reproduced by random pollination within the variety. Each female has the potential to mate with any and all male plants. Open pollination ensures genetic diversity and preserves current traits. An open-pollinated crop must be isolated from outside cannabis pollen. Both male and female plants from the same population are grown together. All males have the possibility to pollinate all females. Heirloom and landrace varieties are open pollinated.
Pollen travels outdoors! Industrial hemp breeders plant seed crops a long way away from other fields of cannabis to avoid contamination from airborne rogue pollen.
Heirloom varieties and seeds are cultivars that were grown before 1950 and are not used in modern, large-scale growing. Heirloom is a term seldom used when referring to medicinal cannabis varieties, because they were developed after 1950. Heirloom varieties retain their traits via open pollination and breed fairly true, producing plants very similar to the parent plants. Weak and weird plants are culled from the population. Heirloom varieties are still seen in Morocco and other hashish-producing regions in other parts of the world.
A landrace is a localized variety of a plant that has developed mainly by natural processes. The plant adapts to the microclimate in which it lives. Landrace varieties are not deliberately or selectively bred to achieve a specific goal. Overall landrace varieties are more genetically and physically diverse than selectively bred plants. Many selectively bred plants started out as landraces and are attempts to improve the gene pool.
Phenotype is the outward physical characteristics or genetic traits expressed that can be observed or measured in cannabis. The phenotype is how the genetic map is expressed as acted on by any environmental factors, internal or external, that occur outside the nuclear envelope.
For example a genotype can be short or tall, but if it grows in a low-light environment, either phenotype could grow tall. If the plant is abused, suffers cold temperatures and heavy winds a plant that would normally grow tall, may be short. Environmental factors can influence the expression of genetic qualities.
The same plant grown indoors will look completely different outdoors when given a chance to express traits. Harsh environmental factors also change phenotype expression. For example, cutting the top off a plant changes the genetic expression and the plant grows short and bushy. The genes are the same but the outward appearance of the plant has changed.
All phenotypes are the observable result of genes acting within the cells of the plant. Sometimes a single gene controls one trait (monogenic traits), and sometimes sets of genes operate together and contribute to make what we see as a phenotype (polygenic traits).
Genotype is the inherited set of instructions carried in a plant’s genetic code. Not all cannabis plants with the same genotype look the same because appearance is modified by environmental conditions (phenotype). And all cannabis plants that look alike do not necessarily have the same genotype.
Environmental conditions often trigger genotypes to express themselves differently. Green leaves often turn purple when exposed to cold temperatures. But leaves on other plants remain green no matter how cold it gets. The plants possess different versions of the gene(s) that control purple pigment production on leaves. These gene versions, alleles, dictate whether or not purple pigment is produced.
In the beginning, both plants had the green-leaved phenotype; one of the plants developed an altered phenotype (purple leaf) in response to the cold environment. It was caused by the plant’s genetic trait interacting with the environment.
A simplistic way to think of this important concept is:
phenotype = genotype + environment
Chemotype was a term coined in 1968 by Dr. Rolf Santesson and his son Johan Santesson. A chemotype is a chemically distinct entity in a plant with differences in the composition of the secondary metabolites. Minor genetic and environmental changes have little or no effect on plant growth or appearance but may produce large changes in the chemical phenotype. Cannabis chemotypes may be indistinguishable in appearance, but differences in chemotypes appear to affect the existence and percentage of cannabinoids and essential oils.

Male and female plants are grown together in this landrace stand of cannabis. The seeded plants are growing in drought conditions.


Two different genotypes of ‘Blueberry’ × ‘Sandstorm’ grown indoors demonstrate different color and growth habits.
Here is the “Classification of Chemotypes” Mel Frank made in the Marijuana Grower’s Guide (1982):
Type Cannabinoid Profile
I high THC—low CBD
II high CBD—moderate to high THC
III high CBD—low THC
IV produce propyl cannabinoids in significant amounts (over 5 percent of total cannabinoids) form a fourth group from both type I and II plants
A multiline variety is a mixture of 2 or more pure lines of similar phenotype with slightly different genes that are resistant to different qualities such as disease, pest and drought resistance, and early maturity. Multiline varieties of the same or closely similar genotypes are also called isogenic lines. The varieties are grown and bred separately but are later mixed together and sold in the same seed package. The diverse genetics often provide extra disease- and pest-resistance or more acclimatization in inconsistent environment.
In a perfect growing year, a gardener may not reap the highest yield from a multiline as is possible with a single hybrid variety suited to the climate, but the degree of variation present in a multiline helps to ensure that as many plants as possible are harvested. This sophisticated breeding technique is common in vegetable and agricultural crops but has yet to be developed for medicinal cannabis varieties.
A synthetic variety is developed by intercrossing several different genotypes that are known to give superior hybrid performance when crossed in all combinations. Synthetic varieties have exceptional hybrid vigor and produce outstanding seed. Synthetic varieties are maintained by open pollination and often recurrent selection in future generations.
(By contrast, a variety developed by mass selection is made up of genotypes bulked together without having undergone preliminary testing to determine their performance in hybrid combination.)
In single-seed descent, a plant is self-fertilized, and the resulting seed is collected. One of these seeds is selected and grown, again self-fertilized, and seed produced. All progeny and future generations have descended from a single ancestor, as long as no pollen from an external family is introduced. Each generation is the result of selfing one individual from the previous generation.
Recurrent selection refers to any breeding program designed to concentrate favorable genes scattered among a number of individuals by repeated cycles of selection for favorable traits.


Two terminal buds from Mel Frank’s ‘Kush #4’ (1977) demonstrate different phenotypes. The first was grown outdoors under harsh conditions—2,000-foot elevation, very hot days and cold nights, and brutal sun. The second phenotype was grown in his Oakland greenhouse with moderate temperatures and filtered sunlight. (MF)
Modern Cannabis Breeding
Modern plant breeding may use techniques of molecular biology to select desirable traits of individual plants or a population of plants. When biotechnology or molecular biology is applied to the plant selection process, it is called molecular breeding.
Molecular markers or DNA fingerprinting can map thousands of genes. Using the process of marker-assisted (or aided) selection (MAS) based on DNA/RNA (deoxyribonucleic acid/ribonucleic acid), variation is used by scientific breeders as an indirect selection tool.
Marker-assisted selection allows breeders to screen large populations of plants for disease resistance, stress tolerance, productivity, and so on. Screening is based on the presence or absence of a specific gene or genes rather than relying on visual observation of a trait in the plant. In MAS, DNA markers are used instead of visual observation to select phenotypes. Marker-assisted selection significantly accelerates the selection process and makes it possible to grow fewer plants for selection. The process can be used at any time in the plant’s life cycle. Plants can be tested in the vegetative growth stage and do not need to flower.
Breeding using molecular biology and marker-assisted selection is more complex than it may appear at first glance. This process enables breeders to measure, select, and cross specific alleles found within the genome. Taking a closer look at MAS, we find that it involves the selection of individuals with specific alleles for traits controlled by a limited number of loci (up to 8).
Marker-assisted backcrossing (MABC) refers to the transfer of a limited number of loci (e.g., transgenes, disease resistance loci, etc.) from one genetic background to another.
Marker-assisted recurrent selection (MARS) involves the identification and selection of 20 or more genomic regions.
Historically, most genetic research for agriculture and commercial flower and vegetable crops has been conducted in agricultural university laboratories in the USA and other countries. Many universities worldwide with strong agricultural departments have the necessary equipment for conducting DNA and RNA tests required for a scientific Marker-Assisted Selection (MAS) breeding program.
Until the last decade, limited marker-assisted selections were employed in cannabis breeding programs. Only a few large companies, such as GW Pharmaceuticals, had the capability to use MAS in their breeding programs to target specific cannabinoids. However, recent developments have made molecular biology technology more accessible and legal in various parts of the world. Setting up a small home laboratory for these purposes has become less expensive over the years.
Laboratories in medical cannabis states and other countries might be open to collaborating with medical cannabis breeders for MAS testing. Agricultural university laboratories may also be interested in conducting MAS testing for cannabis. Some specialized laboratories in the USA, such as ACGT, Inc. and Life Technologies, have experience with molecular plant breeding and have worked with cannabis.
Genetic modification of plants involves adding specific genes to a plant or using RNA interference (RNAi) to knock down a gene, resulting in a desirable phenotype. However, these processes are expensive and have not been perfected for use in cannabis.

To make breeding selections, Jaime from Resin Seeds uses all of his senses and years of knowledge, along with scientific tests.

