Water is a part of humans and all living things. Water moves the fluids that support life in plants and animals. Water (H2O) exists in liquid, gaseous, and solid states. When pure, water is tasteless and odorless. Reflected light often gives it a blue appearance, especially when concentrated in glaciers. Many substances, including fertilizer salts, readily dissolve in water, which is known as the universal solvent. Most sources of water are rarely pure. The impurities in water are “invisible” once dissolved and part of the solution.
Hard Water
Hard water contains significant quantities of dissolved minerals, with calcium (Ca) and magnesium (Mg) concentrations typically used as indicators of its hardness. When hard water is heated, the carbonates formed by the reaction of carbon dioxide (CO2) with bases precipitate, leading to the formation of scale on plumbing pipes, showerheads, and similar surfaces. In hard water, soaps do not lather effectively because they react to form calcium or magnesium salts, which are insoluble. Water with a calcium carbonate (CaCO3) concentration of 100 to 150 milligrams per liter (mg/l) is considered acceptable for growing cannabis. However, water can have 400 ppm (parts per million) of sodium and still be considered soft, but it becomes hard when it contains about 60 to 120 ppm of calcium (depending on the scale used).

A mountain stream in Vancouver, BC, is an excellent source of fresh, clean water. But most water sources available to gardeners are far from pristine.

Salt has started to accumulate on this showerhead. The buildup is from the dissolved solids (salts) in the water supply. The same salts also deposit in soil and containers.

This simple filter removes particles and chlorine from the water supply.

Soft water makes soap lather.
For much more information on hard water, search “hard water” on the www.marijuanagrowing.com forum.
Soft Water
Softened water is often treated with sodium, which binds to the calcium and magnesium ions, removing their ability to form scale or interfere with detergents. In Spain, we have hard water; we put sodium (granular salt) in the dishwasher so spots (scale) will not form on dishes. You can taste the sodium in softened water. Water softeners are used to lengthen the life of plumbing pipes, pumps, and so on, and to increase the effectiveness of soaps. Soft water contains less than 50 milligrams of calcium per liter (50 ppm) and should be supplemented with calcium and magnesium. Sodium in excess of 50 ppm is detrimental to cannabis growth.**
The sodium ions exchange places with a chemical the sodium is attached to, pulling out all multivalent ions (Ca2-, Mg2-) with a similar number of monovalent charges (Na-): every Ca that binds releases two Naions because of the charge difference, a two-for-one deal (in ppm, 100 ppm Ca removed buys 200 ppm Na in return).
A better alternative is often potassium, but it is a little more expensive. Both potassium and sodium exist as the resin used in water softeners and both are monovalent ions—potassium (K-) and sodium (Na ). Potassium is a better option for cannabis and humans. Sodium is economical and widely available, but potassium is more effective.
The metric system facilitates the measurement of “dry residue per liter.” Measure the dry residue per liter by pouring a liter of water on a tray and allowing it to evaporate. The residue of dissolved solids that remains after all the water evaporates is the “dry residue per liter.” The residue is measured in grams. Try this at home to find out the extent of impurities in your water. Fertilizers have a difficult time penetrating root tissue when they must compete with resident dissolved solids, especially sodium. Water that is loaded with high levels of dissolved solids (salts in solution) is possible to manage but requires different tactics. Highly saline water that contains sodium will block the uptake of potassium, calcium, and magnesium. Salt-laden water will always cause problems. If water contains 300 ppm or less dissolved solids, allow at least 25 percent of the irrigation water to drain out of the bottom of containers with each watering. If raw water contains more than 300 ppm of dissolved solids, use a reverse-osmosis device to purify water. Add nutrients to pure water as a way to avoid many nutrient problems.
If raw water contains more than 300 ppm of dissolved solids or more than 50 ppm sodium (Na), use a reverse-osmosis device to purify water before using it in the garden.
Built-up dissolved fertilizer salts often become toxic in container gardens. Excessive salts inhibit seed germination, burn the root hairs and tips or edges of leaves, and stunt plants. Leach excess salt buildup from growing mediums by applying 3 gallons of water per gallon of medium and repeat leaching using a mild pH-corrected fertilizer solution. Leach the growing medium every 2 to 4 weeks to avoid toxic buildup. Hard water and well water in dry climates are often alkaline, and usually contain notable amounts of calcium and magnesium. Cannabis uses large quantities of both nutrients, but too much calcium and magnesium can build up in soil. In general, water that tastes good to people also tastes good to cannabis.
Minerals dissolve into groundwater from rock and sediment. Water sources in low-rainfall or desert regions contain relatively high levels of dissolved mineral salts. For example, Southern Spain and Italy, southwestern USA, and much of Mexico all have high levels of dissolved mineral salts in their groundwater. More than 85 percent of the gardens in the USA have hard water. Many rivers and streams in Alaska, the Great Lakes region, and Tennessee have moderately hard water. Hard and very hard water flows in almost every state in America. Streams in Arizona, Kansas, New Mexico, and Southern California have the hardest water, with dissolved minerals more than 1,000 ppm.
Soft water flows in many parts of Hawaii, New England, the Pacific Northwest, and the South Atlantic and Gulf states. To get an idea of how hard the water is in different parts of the USA, visit www.qualitywatertreatment. com/city_water_guide.htm.
If your water is soft: pH continues to drift up in soft water and purified water with few dissolved mineral solids (less than 60 ppm) due to little or no buffering capacity. Remedy this problem by stabilizing the pH and adding soluble calcium and magnesium, sold at hydroponic stores under the name “Cal-Mag.”
If your water is hard: Treat it with the best option: reverse osmosis (RO).
If water is acidic or alkaline: If EC is low, acidity or alkalinity are weak and will not cause problems. If acidity or alkalinity are driven by multivalent ions, they will directly add an H proton or OH (compounds that do not change things by association). For example, an organic acid is weak and requires less added hydroxide to neutralize, versus phosphoric acid, which requires more hydroxide to neutralize.
Sources of Water
Air conditioner water: Water condensed from an air conditioner or dehumidifier is very clean—virtually no dissolved solids. But the water does trap cannabis fragrance. Most A/C units generate 2 to 3 gallons (7.6–11.4 liters) of water a day. Empty containers on a daily basis!
Rainwater: You can harvest 600 gallons of rainwater from 1 inch of rain that falls on a 1,000 sq ft (93 m2 ) roof. Although slightly acidic in urban areas, rainwater is free of chlorine and pollutants or salts that normally occur in groundwater.
Clean rainwater is an excellent choice for irrigation. Collect runoff by placing a barrel under a downspout. Mix the rainwater with tap water to dilute dissolved solids. Roofs and terraces can accumulate trash, which will pollute the otherwise clean rainwater. Covering your catchbarrel will prevent evaporation and keep out trash. To make sure it is not too acidic (acid rain) and harmful to plants, take pH and parts per million (ppm) readings from collected rainwater before using.
Rivers and streams: Usually, these water resources are publicly controlled. Alpine watersheds supply “mineralized” water—water with elements and nutrients that plants need to produce food and grow.
Tap water: Household water often contains chlorine and other dissolved minerals. Check two or three times a year with your local water bureau to find out what is in your water. Also check the pH regularly. See discussion above about water sources.
| ppm | mg/L | μg/L |
| 100 | 100 | 1,000 |
| 200 | 200 | 2,000 |
| 300 | 300 | 3,000 |
| 400 | 400 | 4,000 |
| 500 | 500 | 5,000 |
| HARD/SOFT WATER INDEX | mg/L | gpg |
| soft | 0–60 | 0–35 |
| moderately hard | 61–210 | 3.5–7 |
| hard | 121–180 | 7–10.5 |
| very hard | 181 | 180 |
| mg/L = milligrams per liter | ||
| gpg = grains per US gallon |

Piping water into the garden facilitates irrigation.

Distilled water is expensive and is best used in small amounts such as for watering cuttings and seedlings.
Clean your tap water by filling barrels and setting them 2 to 3 feet (61–91.4 cm) above the ground. Add ammonium sulfate to settle out the sodium, then siphon water from the top of the barrel, refilling after each watering to allow the chlorine to evaporate. Chlorine, like sodium, is beneficial in small amounts. It is essential to the use of oxygen during photosynthesis and is necessary for root and leaf cell division. But too much chlorine causes leaf tips and margins to burn and leaves to turn a bronze color. Chlorine (which evaporates) and chloramine (which must be filtered to remove) are added to household water systems to kill bacteria, parasites, and other organisms. But both oxidize iron, manganese, and hydrogen sulfide, making them easier to filter out. Empty the barrel periodically, and scrub out residues and sediments. See “Chlorine (Chloride)” in chapter 21, Nutrients, for more information.
Well water: Ground water is pumped from a well. Have your well water analyzed at least once a year because mineral content often changes with the seasons and over time. Do not assume that the mineral content will be the same as that of your neighbors’ well water. Most often well water is hard, with high levels of calcium and magnesium.
Purified Water
Bottled water enjoys minimal regulation in most countries. The US federal government for example, requires that bottled water be at least the same quality as tap water, but some studies show it is of lower quality. Frequently sold as “mineral water” for $1 to $4 USD per gallon, bottled water may contain more dissolved solids than tap water. If you are using bottled water, read labels carefully to ensure that it contains less than 150 ppm (15 mg/L) dissolved solids (aka minerals).
Carbon filters are effective at removing chlorine, chloramines, sediment, and volatile organic compounds (paints, petroleum solvents, and hazardous wastes) from water. But they do not remove dissolved mineral salts from water. Use carbon filters as a prefilter to reverse osmosis (RO) filters.
Deionized (aka demineralized) water has had its mineral ions removed. A water deionizer moves water through special ion exchange resins, complex sodium salts. These resins bind to the mineral dissolved solids (salts), filtering them out of the “pure” water. Deionized water is similar in purity to distilled water. Deionization does not specifically remove viruses or bacteria.
Distilled water has many of its impurities eliminated via distillation, a process that boils water. The resulting vapor is captured and condensed into clean water. Purchasing distilled water is very expensive: $0.75 to $1 USD per gallon. But home distillation systems can cut prices to $0.25 USD per gallon. Distilled water is available at most grocery stores and home improvement centers. Gardeners often use distilled water for cuttings.
Electrodialysis-filtered water is most economical to use in large- and medium-scale installations when desalinating brackish water and seawater. Smaller systems are also available. This process is most efficient when removing ionic components with a low molecular weight.
Water microfiltration systems remove suspended solids down to 0.1 micrometers in size. Use microfiltration as a pre-filter to RO filters to extend the life of RO filters.

European bottled water has the guaranteed analysis printed on the label, but in the USA, no specific analysis is available on the label. The dissolved solids in this bottled water are measured in milligrams per liter (mg/L).
Reverse Osmosis (RO) Water
Reverse-osmosis machines are used to separate dissolved solids from water. These machines move the solvent (water) through the semipermeable membrane. The process is accomplished by applying pressure to the “tainted” water to force only “pure” water through the membrane. The water is not totally (EC of zero), but most of the dissolved solids are removed. The efficiency of reverse osmosis depends on the type of membrane, the pressure differential on both sides of the membrane, and the chemical composition of the dissolved solids in the tainted water. Unfortunately, common tap water often contains high levels of sodium (Na), calcium (Ca), alkaline salts, sulfur (S), chlorine (Cl) and other minerals. The pH could also be out of the acceptable 6.5 to 7 range.
Sulfur is easily smelled and tasted in water. Saline water is a little more difficult to detect. Water in coastal areas is generally full of salt that washes inland from the ocean or sea. Dry regions that have less than 20-inches (50.8 cm) annual rainfall also suffer from alkaline soil and water that is often loaded with alkaline salts.

This reverse osmosis machine transforms water with a high ppm or EC into “clean” water with less than 10 ppm.
pH
The pH scale, from 0 to 14, measures acid-to-alkaline balance. Zero is the most acidic, 7.0 is neutral or base, and 14.0 is most alkaline. Base is 7.0 or above. Just like the algorithmic earthquake Richter scale, every full point change in the pH scale signifies a tenfold increase or decrease in acidity or alkalinity. For example, soil or water with a pH of 5.0 is ten times more acidic than water or soil with a pH of 6.0. Water with a pH of 5.0 is 100 times more acidic than water with a pH of 6.0, and 1,000 times more acidic than water with a pH of 7.0. With a ten-fold difference between each point on the scale, accurate measurement and control is essential to a strong, healthy garden.
Nutrients are available in a plantsoluble form within a limited pH range. The range of solubility is different for each nutrient. Cannabis grows best in soil within a pH of 6.5 to 7.0. Within this range cannabis can properly absorb and most efficiently process available nutrients. If pH is too low or high, mineral salts settle out into a solid (precipitate) from the nutrient solution. If the pH of the nutrient solution is too low, acidic salts chemically bind nutrients, which the roots are then unable to absorb. An alkaline nutrient solution with a high pH causes nutrients to become unavailable. Toxic salt buildup that limits water intake by roots also becomes a problem. Hydroponic solutions perform best in a pH range a little lower than for soil. The ideal pH range for hydroponics is from 5.8 to 6.8. Some gardeners run the pH at lower levels and report no problems with nutrient uptake.
The pH of the growing medium affects the nutrient-solution range and should be kept at a similar pH level as the nutrient solution. For example, most storebought potting soils are slightly acidic, and rockwool hydroponic mediums are often alkaline. For more information on how pH affects plant growth see “pH” in chapters 18, Soil, and 23, Container Culture and Hydroponics, and “Cultural Problems” in chapter 21, Nutrients.
After repeated irrigation, water or nutrient solution with a pH that is too high or low will change the pH of the growing medium. Raw water with adequate levels of calcium and magnesium and a pH above 6.0 will help keep nutrient solutions from becoming too acidic. Climatic conditions can also affect irrigation water pH. For example, the pH can become more acidic in late autumn, when leaves fall and decompose. Large municipalities carefully monitor and correct water supply pH, and there are few water-quality problems.
Add nutrients to water to create a nutrient solution before measuring pH because nutrients are acidic and will affect the outcome. Once nutrients are mixed in solution, wait a few minutes so that the solution can stabilize before measuring. I like to measure the pH of the original water to get an idea of how much the nutrients acidify the solution. If the pH of water is too low, add soluble potassium bicarbonate to neutralize it. Find potassium bicarbonate, also an organic fungicide, at pharmacies and hydroponic centers. However, water that is high in bicarbonate (HCO3 ) makes it hard to keep the pH down, and it is difficult to avoid scale (mineral buildup) on equipment.
Once tested, the pH should be within the acceptable range for soil or hydroponics (see chart on page 340). Continue to fine-tune the pH level by adding small amounts of chemicals.
Phosphoric acid is the most popular substance used to lower pH. Potassium hydroxide is popular to raise pH. Both of these chemicals are relatively safe, although they can cause burns and should never come in contact with eyes. Most hydroponic supply stores sell easy- to-use pH adjusters that are diluted to a reasonably safe level.
Concentrated pH adjusters can cause large pH changes and can make adjusting the pH very frustrating. The pH will ‘bounce’ up and down when adjusted with a concentrated product when added in very small amounts. Not only does the pH overshoot the desired mark, the availability is virtually destroyed. If using such concentrates, dilute the chemical with a larger volume of water before adding it to the nutrient-solution reservoir.
Altering Nutrient-Solution pH
I like to use pH Up and pH Down from the hydroponic store rather than lessreliable citric acid, baking soda, or vinegar. Avoid using potassium hydroxide and sodium hydroxide, commonly used in hydroponic gardens, because they are caustic and require special handling.
Hydroponic gardeners use phosphoric and nitric acid to lower pH. Calcium nitrate can also be used but is less common. Such acids can be used to lower pH but must be added more often.

Keep the nutrient reservoir aerated to ensure maximum uptake from plants.
Test the pH regularly, at least once a week, to ensure that it stays within the acceptable range for either soil or hydroponics. Water evaporates from hydroponic nutrient-solution tanks and transpires from foliage, using more water than nutrients. Both cause a concentration of nutrients, acidifying the nutrient solution and lowering the pH. The pH and electrical conductivity (EC) of water supplies in municipalities and cities can also change throughout the year.

pH down lowers pH of water or a nutrient solution.
Measure the pH of water and nutrient solutions with litmus paper or an electronic pH tester. Both are available at most nurseries, home improvement centers, and hydroponic stores. Comparing the color of the soil/chemical mix to the color of the chart can be confusing. If you use one of these kits, make sure to buy one with good, easy-to-read color codes. Follow instructions supplied by the manufacturer.
Electronic pH testers are economical and convenient. Less-expensive pH meters are accurate enough for casual use. More-expensive models are very accurate. I prefer electronic pH meters to reagent test kits and litmus paper because pH meters are convenient, economical, and accurate. Once purchased, you can measure pH thousands of times with an electronic meter, while the chemical test kits are good for about a dozen tests. Perpetual pH-metering devices are also available and are most often used to monitor hydroponic nutrient solutions.
For an accurate pH test with an electronic pH meter:
- Clean the probes of the meter after each test and wipe away any corrosion.
- Pack the soil around the probes.
- Water soil with distilled or pH-neutral water before testing.
- Use a dilution test of one part growing medium to one part distilled water. Stir, allow to sit, and then drain away liquid into a separate cup, using a filter for the medium, and then measure the water.


Add nutrients to the water first, and then check the pH. For example, the pH of the image on the left started out at 8.4, which is very high. Once the (acidic) nutrients were added, the pH dropped after an hour in solution. The gardener had to use pH down to drop the pH one more point to 6.4.

This beautiful crop of ‘Jolly Bud’ grown by DoobieDuck has no trouble with nutrient or water uptake.
Raising Nutrient-Solution pH
Ammonium carbonate (NH4)2CO3, aka “bakers’ ammonia,” is nitrogen in the form of ammonia, but it is not a good choice to alter pH. When crushed it is used as smelling salts, and yes, it smells bad!
Calcium hydroxide (Ca(OH)2, aka hydrated lime, builder’s lime, or pickling lime) is an inorganic compound. Use only in small amounts because it is soluble and very fast-acting.
Potassium bicarbonate (KHCO3) works well to neutralize or buffer pH. It is a common ingredient in club soda and is used as a source of CO2 in baking and in dry chemical fire extinguishers, among other things. Potassium bicarbonate also works as an organic surface fungicide for powdery mildew. Be careful, concentrations above 0.5 percent can have toxic effects on plants.
Potassium carbonate (K2CO3) is a common ingredient in pH up solutions and it works well to raise pH in nutrient solutions. It is used as a leavening when baking gingerbread and as a buffering agent in wine production. It is also used for fire suppression.
Potassium (potash) silicate (K2SiO3) will raise pH quickly and add potassium to the nutrient solution and growing medium. It can be used as a foliar mist. Potassium silicate is also used to make welding rods and as an anticorrosive agent.
Potassium hydroxide (NOH, aka caustic potash) is relatively safe and very popular to raise pH. Potassium hydroxide is also mixed with potassium carbonate to buffer pH when using soft water. This inorganic compound is a strong base with many industrial uses, including cleaning chemicals, the manufacture of biodiesel and batteries.
Sodium bicarbonate (NaHCO3, aka baking soda, bread soda, cooking soda, and bicarbonate of soda) will raise pH. It is also used as a stomach antacid. Note: See “Ammonium carbonate.”
Sodium carbonate (Na2CO3) is a commonly used salt in water softeners. Do not use sodium carbonate to alter pH. It is detrimental to plant growth.
Sodium hydroxide (NaOH, aka lye and caustic soda) is very corrosive. It is commonly used as drain cleaner. Do not use sodium hydroxide to alter pH.
Safety Precautions Dilute pH adjusters so they are safer and more forgiving, and dosages are easier to control.
Carbonates and hydroxides are bases.
Read labels completely and follow instructions.
Keep out of the reach of children.
Keep an ample supply of fresh water to dilute accidental spills.
Wear a mask, rubber gloves, long sleeves, and other protective clothing.
Do not inhale toxic fumes.
Store pH-adjusting agents in an airtight container to guard against spills and accidental activation with moisture. For example, when potassium hydroxide absorbs humidity, it turns into corrosive sludge.
Lowering Nutrient-Solution pH
Why does nutrient-solution pH constantly drop?
It depends on many things, from the medium to the air driven into it or exposed to it. The plant is also giving off protons (H+ ions ) that add to the pH pool and lower the pH. This could almost be another chapter in this book!
The most common reason pH fluctuates is because native water is soft and has no buffering agents to stabilize pH.
Acids are used to lower pH. Most fertilizers are acidic and naturally lower pH. Acids that are not diluted in water are dangerous—they undergo chemical change so readily that they can react with skin and cause burns.
Use extreme caution when handling acids. The higher the concentration, the more corrosive they are. Acids can eat metals and burn your skin!
Hydroponic gardeners use phosphoric and nitric acid to lower pH. Calcium nitrate can also be used but it is less common. Such acids can lower pH but must be added more often.
Ammonium monopotassium phosphate (KH2PO4, aka potassium dihydrogen phosphate, KDP, or monobasic potassium phosphate, MKP) is most effective to raise pH from 5.5 to 7.0. It also buffers pH well when within this range. The buffering ability diminishes below pH 5.5. It is a source of phosphorus and potassium. Gardeners can use ammonium monopotassium phosphate to minimize the escape of ammonia from nutrient solutions and growing mediums. It is also used as a fertilizer, food additive (in Gatorade, for example), and fungicide. The soluble salt can also be used as a fertilizer.
Ammonium nitrate (NH4NO3) is a high-nitrogen fertilizer, and can also be used in explosives.
Ammonium sulfate is often recommended to lower pH because it is difficult to overapply; however, cannabis can tolerate very little ammonium. Avoid using ammonium sulfate.
Calcium nitrate Ca(NO3)2 is also known as nitromagnesite, Norwegian saltpeter, Norgessalpeter, or nitrate of lime. It is an inorganic compound used in concrete.
Citric acid is unstable because plants break it down, and it tends to drift up after a few hours unless well buffered. Citric acid can be obtained from lemons and limes but is most often made from other sources. Citric acid should be used in emergencies only. It can also cause bacteria to grow, lowering dissolved oxygen in the nutrient solution and competing with roots.
Hydrochloric acid, a solution of hydrogen chloride (HCl), lowers pH quickly and efficiently. It is water-soluble. But it is a highly corrosive mineral acid that is used in cleaning supplies, PVC plastic, swimming pool maintenance, and many other products. Not recommended!
Muriatic acid (concentrated hydrochloric acid). Cannabis can tolerate low levels (<100 ppm) of chloride, which is derived from hydrochloric acid. Muriatic acid is produced from hydrochloric acid and common salt, sodium chloride (NaCl).
Nitric acid (HNO3), a major component in fertilizers, lowers pH and does not precipitate when pH is high. It is a highly corrosive, strong, and toxic acid. Use at high dilutions and low concentrations. A concentration of 86 percent or more is called fuming nitric acid and reacts violently (often creating explosions) with many nonmetallic compounds.
Phosphoric acid* (H3PO4, aka orthophosphoric acid or phosphoric (V) acid) is one of the most popular chemicals used to lower pH in hydroponic gardens. Hydroponic stores sell it in dilute mixes that are ready to use and relatively safe. Phosphoric acid changes pH relatively quickly. Avoid using when pH is high and there is an abundance of phosphorus available to plants. Excess phosphorus tends to precipitate. Change to nitric acid in such situations.
Phosphoric acid is also used to “convert rust” to black ferric phosphate; in gel form it is called naval jelly. Food grade phosphoric acid is used to acidify foods and beverages.
Potassium nitrate (KNO3, aka saltpeter or saltpetre) is a food preservative and an important ingredient in gunpowder.
Magnesium nitrate [Mg(NO3)2] has 10.5 percent nitrogen and 9.4 percent magnesium.
Sodium hydroxide (NaOH) Do not use!
Sodium nitrate (NaNO3, aka Peru saltpeter or Chile saltpeter) is used in fertilizer as a source of nitrate, food preservatives, and explosives.
Sulfur (S) is insoluble and unavailable to plants. Till elemental sulfur into soil, and oxygen turns sulfur into SO4, which is readily available for uptake by roots. Most effi cient oxidation takes place to convert sulfur to SO4 in warm weather in lightly moistened, well-aerated soil. Cold temperatures and water-saturated soil slow the conversion. Elemental sulfur works well when applied a few weeks before the growing season. Take care when adding large quantities of elemental sulfur because it can quickly acidify soil.
Ammonium sulfate is an acid-forming material; K-Mag fertilizer, potassium sulfate, and calcium sulfate are neutral materials and have no effect on soil pH.
Active soil bacteria convert sulfur to sulfuric acid, lowering soil pH. The process is slow and soil temperature must be above 55oF (12.8oC). Do not fl ood soil (which creates anaerobic conditions) or else sulfur will convert to hydrogen sulfi de that will kill roots (and smell like rotten eggs).
Ferrous sulfate also decreases soil pH, but it costs more than sulfur, and eight times more of it is needed in comparison to elemental sulfur.
Aluminum sulfate is expensive, and there are reports of aluminum toxicity when too much is applied. It changes soil pH instantly once mixed with soil.
Ammonium sulfate and sulfur-coated urea fertilizers have little effect on pH. For example, ammonium sulfate fertilizer 21-0-0 at 10 pounds per 1,000 square feet (92.9 m2 ) can change soil pH from 7.5 to 7.4.
Iron sulfate is soluble but 6 times more of it is necessary than elemental sulfur to change pH. It reacts in 3 to 4 weeks— faster than elemental sulfur—but can damage roots.
Magnesium sulfate (MgSO4) contains magnesium, sulfur, and oxygen. Soluble Epsom salts (MgSO4·7H2O) work well to correct magnesium deficiencies in soil and cannabis plants.
Sulfuric acid (H2SO4) is sold as swimming pool and car battery acid at most grocery stores, by the quart or liter. Mix 1 cup with a gallon of distilled water and get a gallon of pH down for about $1 USD. It also adds sulfur to the mix. Battery acid is 40 percent sulfuric acid. Do not use toxic battery acid from a battery; it is contaminated with lead!
Vinegar is the product of ethanol fermentation, resulting in acetic acid.
The pH of table vinegar ranges from 2.4 to 3.4. The pH climbs when diluted. Typically, acetic acid ranges from 4 to 8 percent concentration for table vinegar. Vinegar used for pickling can be as high as 18 percent. Cannabis breaks vinegar down, causing pH to climb. Vinegar also causes excess bacterial growth that uses soil oxygen and acidifies soils.
How Fluids Move within Cannabis
Water is essential to plant life. It provides a medium to transport nutrients necessary for plant life and make them available for absorption by the roots. Water quality is important for this process to work at maximum potential. With this in mind, the first question a medical cannabis gardener needs to ask about water is, “What’s in the water, and how does it affect growing cannabis?”
Everything in water can affect how plant roots absorb it.
Microscopic root hairs in the rhizosphere (root zone) absorb water and nutrients in the presence of oxygen and carry them up the stem to the leaves. This flow of water from the soil through the plant is called the transpiration stream. A fraction of the water is processed and used in photosynthesis. Excess water evaporates into the air, carrying waste products along with it via the stomata in the leaves. This process is called transpiration. Some of the water also returns manufactured sugars and starches to the roots.
Roots support the plant, absorb nutrients, and provide the initial pathway into the plant’s vascular system. A close-up look at a root reveals the xylem and phloem core—vascular tissue enveloped by a cortex tissue, or the layer between internal vascular and external epidermal tissue. The microscopic root hairs are located on the epidermal tissue cells. These root hair follicles are extremely delicate and must remain moist. Roots and root hairs must also be protected from abrasions, extreme temperature fluctuations, and harsh chemical concentrations. Plant health and well-being is contingent upon strong, healthy roots.
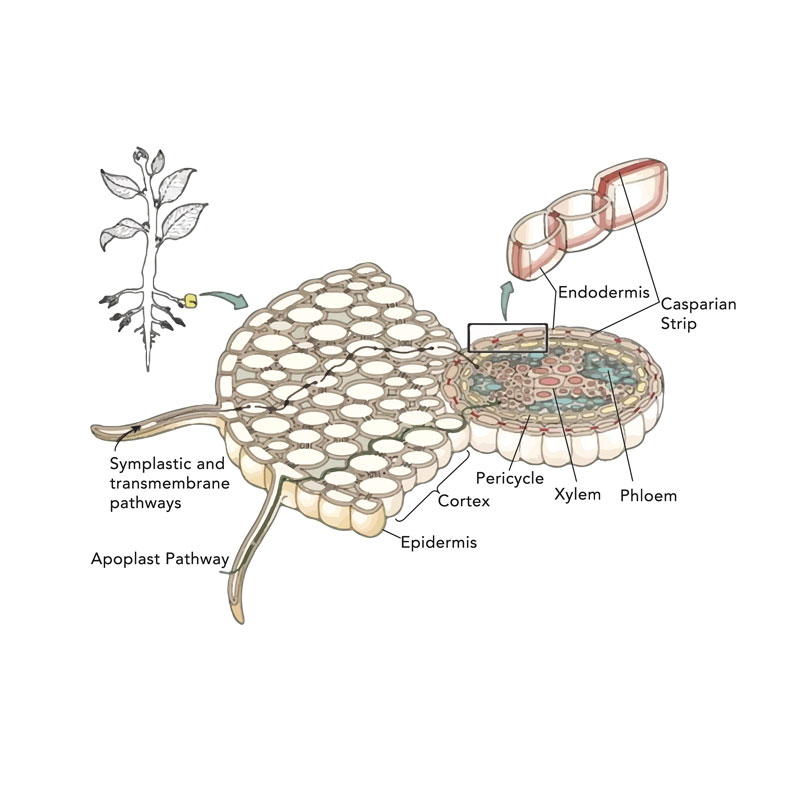
Everything goes through the same pathway, the Casparian strip. This is where the difference in a water root and terrestrial root occurs by thickening.
A majority of nutrient absorption begins at the root hairs, and the flow continues throughout the plant via the vascular system. Absorption is sustained by diffusion, in which water and nutrient ions are uniformly distributed throughout the plant. The intercellular spaces— apoplasts and connecting protoplasm, called symplast—are the pathways that allow the water and nutrient ions and molecules to pass through the epidermis and the cortex to the xylem and phloem’s vascular bundles. The xylem channels the solution through the plant while phloem tissues distribute the food manufactured by the plant. Once nutrients are transferred to the plant cells, each cell accumulates the nutrients it requires to perform its specific function.
The solution that is transported through the vascular bundles or veins of a plant has many functions. This solution delivers nutrients and carries away waste products. It provides pressure to help keep the plant structurally sound. The solution also cools the plant by evaporating water via the leaves’ stomata.
Osmosis
Water and molecules or atoms smaller than a water molecule can move through the semipermeable membrane. All other stuff enters through various ports and gates and controlled entry and transport. Water is given almost free reign, which is why a plant can recover from wilt so quickly when dry, but it takes three days for nitrogen to reach the right place in the plant. But flow can go the other way, too; it is all based on the principle of equilibrium, that a solution will achieve an equal concentration or disbursement of the ions dissolved in it. The solution is separated, the potential still exists, and water molecules are what move to dilute the more concentrated areas when the ions cannot move because of a barrier, in this case a semipermeable barrier through which only water molecules can pass. So water moves into the plant by a difference in the potential between the area of higher concentration (plant tissue) from a lower concentration (soil solution). If the potential is higher outside the plant tissue, such as overfertilization, water will move backward, governed by the laws of equilibrium again, from the plant into the soil, and thus burns are caused on the upper tissues.
The roots draw the water up the plant by osmosis, the process by which fluids are drawn through a semipermeable membrane and mix with each other until the fluids are equally concentrated on both sides of the membrane. Semipermeable membranes located in the root hairs allow specifi c nutrients that are dissolved in the water to enter the plant while the other nutrients and impurities are excluded. Since salts and sugars are concentrated in the roots, the electrical conductivity (EC) inside the roots is (almost) always higher than that outside the roots.
Here is a simplistic view of how osmosis works: It depends on the relative concentrations of each individual nutrient on each side of the (root) membrane; it does not depend on the total dissolved solids (TDS) or EC of the solution. For nutrients to be drawn in by the roots via osmosis, the strength of the individual elements must be greater than that of the roots.
Osmosis does depend on solution EC on either side of the root. However, the ions are allowed into the cell that may have a root hair by a larger pore structure of the membrane and is pulled in by the flow of water molecules from the soil solution into the plant. They may even be able to continue this pathway to the core, but will be switched over at the Casparian strip. In reality, for elements to enter apoplastically, they have to be smaller not stronger.
But, the transport of water (instead of nutrients) across the semipermeable membrane depends on EC. For example, if EC is greater outside the roots than inside, plants dehydrate as water is drawn out of the roots. In other words, salty water with a high EC can dehydrate plants.
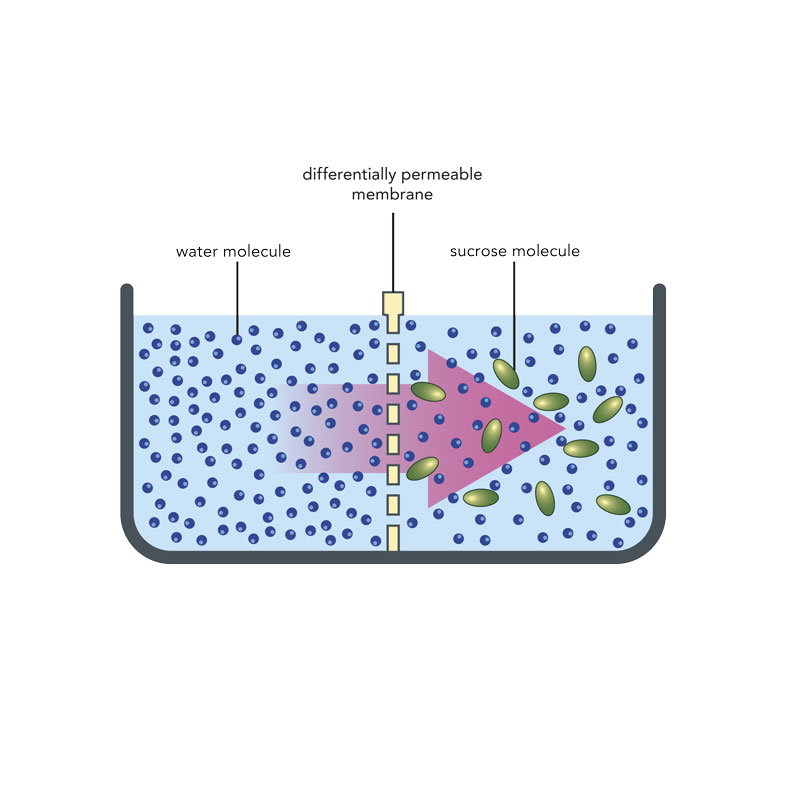
This simple drawing shows the basic principle of osmosis on a molecular level. When the concentration of “salts” is greater on one side of a barrier such as a cell wall in a plant, the salts migrate to the other side to equalize the pressure.
Irrigation
Having a readily accessible water source is convenient, and it saves time and labor. For example, a 4 × 4-foot indoor garden of 16 healthy plants in 3-gallon (11.4 L) pots needs 10 to 25 gallons (37.9–94.6 L) of water per week. Large outdoor plants in big containers can use 5 to 10 gallons of water daily. Water weighs 8 pounds per gallon (1 kg/L). That’s a lot of containers to fill, lift, and spill. Carrying water in containers from the bathroom sink to the garden is okay when plants are small, but when they are large, it is a big, sloppy, regular job.
Irrigation guidelines:
- Water plants in containers when they are half-full of water; weigh pots to tell the difference.
- Water soil gardens when the soil is dry one-half inch (1.3 cm) below the surface.
- Water containers with a mild nutrient solution and let 10 to 20 percent drain off at each watering.
- Do not let soil dry out to the point that plants wilt.
- Do not let roots sit in water, such as a saucer, for more than 20 minutes at a time or roots will drown.

This drip system in a greenhouse uses two feeder hoses for each large container to ensure that the entire soil mass receives adequate irrigation.

Plant in the earth outdoors or in a greenhouse.

This outdoor garden is protected from wind by white walls that also reflect light. The pallets under pots allow for better drainage and keep soil up off the cold concrete floor, which keeps them warmer.
Large plants use more water than small plants, and small containers must be watered more often than large containers. But many more variables than plant or container size dictate a plant’s water consumption. The health, age, variety, and size of the plant, along with its container size, soil texture, temperature, humidity, ventilation, and the intensity of wind and light, all contribute to water needs. Changing any one of these variables can alter the plant’s water consumption. Good ventilation is essential to promote a free flow of fluids, transpiration, and rapid growth. The healthier a plant is, the faster it grows, and the more water it needs.
In general, sativa varieties have a more extensive root system and use more water than indica varieties.
Small plants with a small root system in small containers of soil must be watered frequently—as soon as the soil surface dries out. If exposed to wind, small plants will dry out very quickly.
Irrigate soil and soilless mixes when they are dry one-half inch below the surface. As long as drainage is good, it is difficult to overwater fast-growing cannabis. Four-week-old clones flowering in 2- to 3-gallon (7.6 to 11.4 L) containers need to be irrigated once or twice daily. In fact, most gardeners prefer smaller containers because they are easier to control.
Flowering cannabis uses high levels of water to support rapid floral formation. Withholding water can stunt flower formation. Plants exposed to wind tend to dry out much faster than those that are sheltered, so it’s important to provide appropriate protection and adequate watering during the flowering stage.
Mulching the top surface of the soil helps conserve water and prevents the formation of a crust. However, it’s important to note that mulch can sometimes make it difficult for irrigation water to penetrate evenly. When irrigating, ensure that water penetrates the mulch layer uniformly to reach the soil beneath.
Outdoor, terrace, and patio plants can consume significantly more water, up to three or four times their normal usage, on hot, windy days. Keeping up with watering can be challenging and time-consuming. To mitigate the impact of wind on plants, consider using an automated watering system or providing a windbreak. Applying mulch can also reduce soil evaporation. When watering, use an ample amount of water and allow for up to 10 percent runoff during each watering session to prevent fertilizer buildup in the soil. It’s advisable to water early in the day, allowing excess moisture to evaporate from the soil surface and leaves. Avoid leaving foliage and soil wet overnight, as this can invite fungal attacks.
If planting in the earth outdoors, competing plants, especially established trees and bushes, suck up irrigation, depriving annual cannabis plants. For example, in my backyard, the oak trees on the other side of my fence suck up so much water that I must give the cannabis plants 3 to 4 times as much irrigation water as the same varieties in raised beds that have no competition from trees.
Sometimes the soil surface down as far as 6 inches (15.2 cm) is moist, but below the wet mark, soil may be bone dry. But the opposite may be true too. Fine topsoil and compost on the soil surface dry rapidly during windy days with a lot of sun and the soil below could remain super wet.
Moisture meters take most of the guesswork out of irrigating cannabis. They can be purchased for less than $30 USD and are well worth the money. Moisture meters measure exactly how much water the soil contains at any level or point. Often the soil will not hold water evenly, and thus it develops dry pockets. Checking the soil moisture with a finger provides an educated guess but disturbs the root system. A moisture meter will give an exact moisture reading without disturbing the roots.
Use a moisture meter outdoors to test the growing medium moisture at different depths. For example, if you continue to water plants and the water accumulates below the soil surface, oxygen could be squeezed out of the soil. However, the soil structure plays a major role. Porosity and the mix of appropriate size (large and small pore space) and any blocking actions in the mineral soil can change the equation. Water moves initially through the soil in all pore spaces while gravity pulls it through, and it is picked up by surrounding small pores through capillary action. It vacates the large pores under gravity and occupies the smaller. If the pores are all small, the amount of time to drain can increase; if the pores change size suddenly then the flow can be blocked such as the famed Texas clay pan; and if the amount of clay in the mineral soil is high, it can be smoothed during a preparation known as glazing, which stops the flow of water. But either way or mechanism, it is not because the water is heavy, it is because the water accumulates for a given reason, indicating blocked or restricted passage.
50 Percent Watering Rule
Weigh a container after watering to determine its “full” weight. Weigh the container again after several days when it reaches “half” its weight to find the 50 percent full weight. Water container plants when they reach 50 percent of their weight. For instance, a plant in a 3-gallon (11.4 L) container weighs 2.2 pounds (1 kg) when fully watered and 1.1 pounds (499 gm) when it’s 50 percent full. It’s time to water when the container weighs 1.1 pounds (499 gm).
One of the biggest issues with soils that dry down is that all the salts that are in solution adhere to the growing medium particles when the water disappears. As soon as water is reapplied, no matter at what EC, all of these salts, as well as any associated with the soil particles, immediately go into solution. For example, if the medium has an EC of 4.0 when dry, and very clean RO water is applied, the water will, for a short time until equilibrium is obtained, have an EC of 4.0, causing salt burn. If the EC is at 2.0 and the medium dries and watering is done at 1.6, then the EC jumps to 3.6, immediately causing issues. The solution to the problem is to run water into the container several times, which allows the medium to rehydrate and wash out the salts, then reapply fertility at normal values. Of course, this must all occur within the 20-minute time period.

Fill the container to capacity with water.

Weigh the container to determine its water content. When it weighs half as much as it did when fully saturated with water, it’s time to water the plant.

Tip the container to check if it is heavily laden with water. Irrigate the light container.
Cultivate the soil surface to allow water to penetrate evenly and guard against dry soil pockets. Such cultivation also keeps water from running down the crack between the inside of the pot and the soil and then out the drain holes. Using Smart Pots or similar container will also solve this problem. See chapter 19, Containers, for more information. Gently break up and cultivate the top half-inch (1.3 cm) of the soil with your fingers, a salad fork, or a lightweight cultivator. Be careful not to disturb the tiny surface roots. After you develop some skill at knowing when the plants need water, you can check to see how heavy they are simply by tipping them.
Outdoor gardens may need deeper cultivation to aerate soil, but cultivate with care! If soil compacts around plants, insert a garden fork in the soil and wiggle it a little before removing. This will break up the soil and allow air to penetrate. Do not disturb soil much or it will break many roots.
Keep containers lined up in rows when growing and watering. It is much easier to keep track of watered and fertilized pots when they are lined up.
Overwatering
Cannabis does not like soggy soil. Soil kept too wet drowns roots, squeezing out the oxygen. This causes slow growth and possible fungal attack. Poor drainage is most often the cause of soggy soil. It is compounded by poor ventilation and high humidity.
Applying more water to the medium than it can hold against gravity for more than 20 minutes causes overwatering. Overwatering occurs when more water is applied to the growing medium after it has already been saturated for 20 minutes or more, or before the plant needs watering. After 20 minutes roots suffer from lack of oxygen and die.
Overwatering is a common problem, especially with small plants that have little volume in containers. Too much water drowns the roots by cutting off their supply of oxygen. Again, the most important thing to remember is to never let soil be saturated for more than 20 minutes.
If you have symptoms of overwatering, use a moisture meter. Pay attention to the levels of moisture in containers and in outdoor garden soil. Often dry soil pockets develop amidst moist soil. Sometimes, parts of the soil are overwatered and other soil pockets remain bone-dry. Lightly cultivating the soil surface, allowing even water penetration, and using a moisture meter will help overcome this problem. Indoors and in greenhouses, one of the main causes of overwatering is poor air ventilation. Plants need to transpire water into the air. If there is nowhere for this humid air to go, gallons of water are locked in the air of the enclosed area. Well-ventilated air carries this moist air away, replacing it with fresh, dry air. If you are using trays to catch runoff water, use a turkey baster, large syringe, or sponge to draw the excess water from the tray so that plants do not sit in stagnant water.

Inexpensive moisture meters help take the guesswork out of irrigation.

Heavy clay soil does not drain well and stays soggy for a long time. Soil or soilless mixes that drain well are essential to rapid cannabis growth.

One sure sign of overwatering is when leaves curl under on the edges.
Signs of overwatering include:
- Leaves curled down and yellowed.
- Waterlogged and soggy soil.
- Fungal growth.
- Slow growth.
Symptoms of overwatering are often subtle, and inexperienced gardeners may not see any blatant symptoms for a long time.
Overwatering at the end of June, followed by a cold snap in my friend Nomaad’s garden, retarded plant growth by two weeks. Plants had to be harvested later and produced about 20 percent less. If not overwatered, the plants would not have had spotty growth and would have flowered earlier. Pay attention to water temperature, too. If large outdoor pots are too wet at night, conditions are perfect for fungus growth to develop. Irrigate early in the morning so plants have enough time to absorb the water during the day.
Underwatering
Underwatering is less of a problem indoors and in backyards but fairly common if small (1- to 2-gal) pots are used, and the gardener does not realize the water needs of rapid-growing cannabis. Small containers dry out quickly and may require daily watering. If forgotten, water-starved plants become stunted. Once the tender root hairs dry out, they die. Most gardeners panic when they see their prize cannabis plants wilting in bone-dry soil. Dry soil, even in pockets, makes root hairs dry up and die. It seems to take forever for the roots to generate new root hairs and resume rapid growth.

A water wand with a breaker mixes air with irrigation water just before applying.

Add a few drops of biodegradable liquid dish soap concentrate to the irrigation water. The detergent helps water penetrate the soil more thoroughly.

Underwatering causes plants to become stunted and take up water and nutrients at erratic rates. This little plant could have grown much better had the soil surface been cultivated so water could penetrate evenly.

Set up a drip irrigation line to water rows of plants.
Add a few drops (one drop per pint [47.3 ml]) of a biodegradable, concentrated liquid soap like Castille or Ivory to the water. It will act as a wetting agent by helping water penetrate the soil more efficiently, and it will guard against dry soil pockets. Apply about one quarter to one half as much water/fertilizer as the plant is expected to need, and then wait 10 to 15 minutes for it to totally soak in. Apply more water/fertilizer until the soil is evenly moist. Avoid letting runoff water sit in trays for more than 20 minutes after initial watering. Remove excess water with a large turkey baster.
Another way to thoroughly wet pots, especially ones that have fully dried, is to soak the containers in water. This is easy to do with small pots. Simply fill a 5-gallon (18.9 L) bucket with 3 gallons (11.4 L) of water. Submerge the smaller pot inside the larger pot, for a minute or longer, until the growing medium is completely saturated. Wetting plants thoroughly ensures against dry soil pockets. Do not submerge pot for more than 20 minutes or it will kill roots.

Whether in containers or planted directly in the ground, mulch plants (here with straw) to conserve moisture and protect the soil surface.

